Market Overview
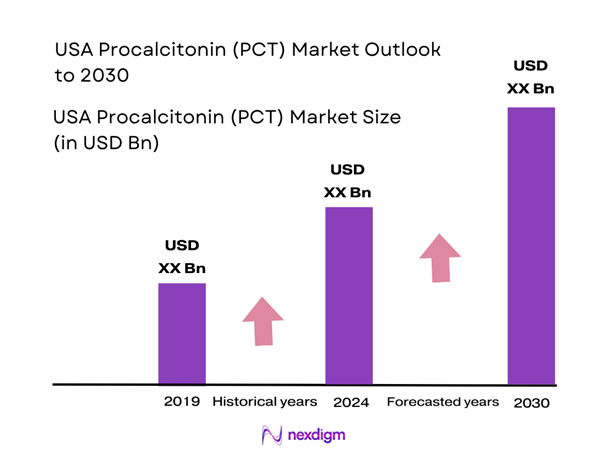
The USA Procalcitonin (PCT) market is valued at USD 1.2 billion in 2024 with an approximated compound annual growth rate (CAGR) of 4.37% from 2024-2030, reflecting a robust growth trajectory driven by the increasing burden of sepsis and respiratory infections in the country. The growing awareness of procalcitonin testing’s benefits and the rising emphasis on early diagnosis among healthcare providers have significantly contributed to the market’s expansion.
Key cities and states, including New York, California, and Texas, dominate the Procalcitonin market due to their high population densities, leading healthcare facilities, and significant investments in healthcare sectors. These states have established healthcare ecosystems, advanced research institutions, and a higher incidence of conditions necessitating PCT testing, making them critical hubs for market growth. Their investments in medical technology and research foster innovation, creating a favorable environment for the PCT market to thrive.
Market access regulations in the U.S. healthcare system also significantly impact the adoption and proliferation of procalcitonin testing. The reimbursement landscape, governed primarily by Medicare and Medicaid services, poses challenges for new diagnostic tests entering the market. It is reported that the Centers for Medicare & Medicaid Services (CMS) regularly evaluates diagnostic tests for their effectiveness and assigns reimbursement levels accordingly.
Market Segmentation
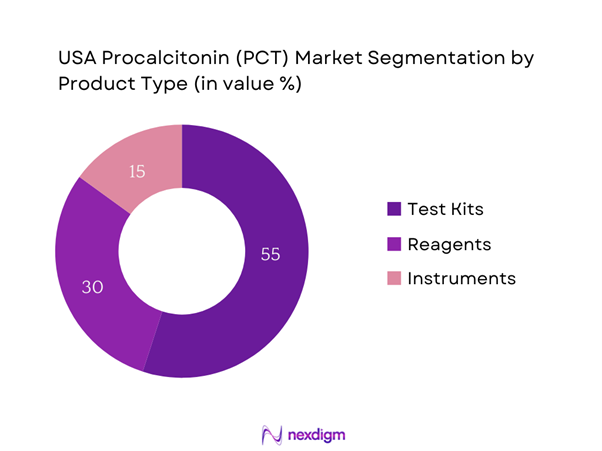
By Product Type
The USA Procalcitonin market is segmented by product type into test kits, reagents, and instruments. Among these, test kits account for the majority market share. The dominance of test kits can be attributed to their critical role in the rapid diagnosis of infections, particularly sepsis, that require immediate intervention. The convenience and efficiency of these kits in delivering results quickly are highly valued by healthcare providers, facilitating timely clinical decisions. Companies are increasingly investing in developing high-quality, easy-to-use test kits that ensure accuracy while accommodating the fast-paced environment of emergency care settings.
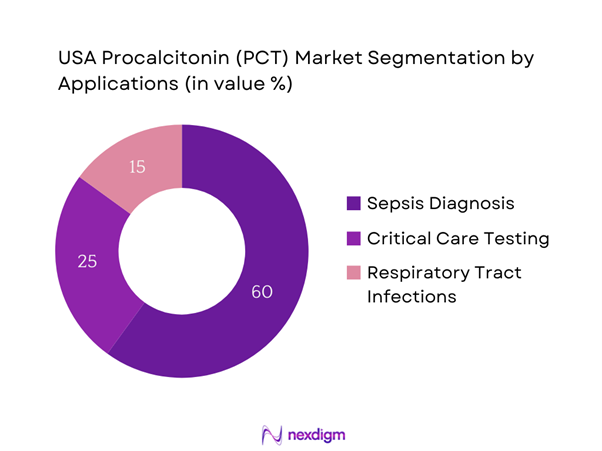
By Application
The USA Procalcitonin market is segmented by application into sepsis diagnosis, critical care testing, and respiratory tract infections. Sepsis diagnosis holds the largest market share, primarily due to the rising awareness of the condition and the need for early detection methodologies. As healthcare providers increasingly recognize the importance of PCT as a biomarker for diagnosing sepsis, the demand for associated testing solutions has surged. This segment benefits from growing educational initiatives aimed at improving the management of sepsis, further driving the market for PCT applications in critical clinical scenarios.
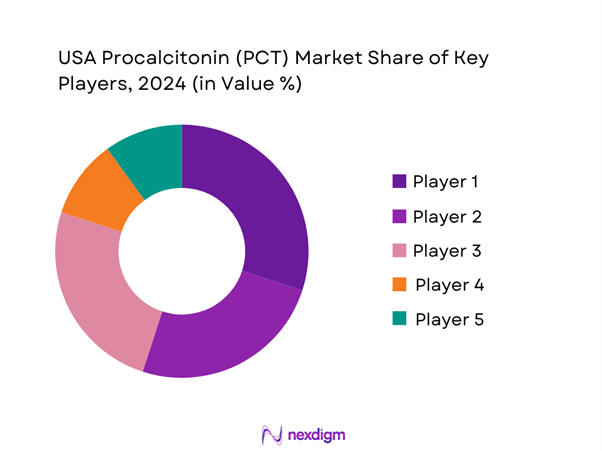
Competitive Landscape
The USA Procalcitonin market is dominated by several key players, including Roche Diagnostics, Thermo Fisher Scientific, bioMérieux, and others. These companies significantly influence the market by offering innovative and reliable diagnostic solutions tailored to meet the demands of healthcare providers. Their established reputations, extensive distribution networks, and consistent investments in research and development highlight the competitiveness in this sector.
| Company | Establishment Year | Headquarters | Test Kits | Reagents | Instruments | R&D Investment | Market Presence | Distribution Channels |
| Roche Diagnostics | 1896 | Basel, Switzerland | – | – | – | – | – | – |
| Thermo Fisher Scientific | 1956 | Waltham, Massachusetts | – | – | – | – | – | – |
| bioMérieux | 1963 | Marcy l’Étoile, France | – | – | – | – | – | – |
| Abbott Laboratories | 1888 | Abbott Park, Illinois | – | – | – | – | – | – |
| Siemens Healthineers | 1847 | Erlangen, Germany | – | – | – | – | – | – |
USA Procalcitonin (PCT) Market Analysis
Growth Drivers
Increasing Incidence of Sepsis
The incidence of sepsis has been rising in the United States, with approximately 1.7 million adults diagnosed with sepsis each year, according to the Centers for Disease Control and Prevention (CDC). This condition is responsible for nearly 270,000 deaths annually, making it a significant public health concern. As the population ages, and with increases in chronic diseases, the demand for diagnostic solutions to detect sepsis early continues to grow. The urgency for quick diagnosis has led to escalated focus on biomarkers like procalcitonin, further driving the market for PCT testing.
Advances in Diagnostic Technologies
Technological innovations in diagnostics are transforming healthcare, with platforms utilizing artificial intelligence and rapid testing solutions gaining traction. The FDA reported that as of 2023, there were over 200 FDA-cleared tests that utilize bioinformatics and Point-of-Care technologies specifically for sepsis detection. Rapid diagnostic tests for procalcitonin offer quicker results, allowing healthcare providers to make more informed treatment decisions promptly. As healthcare systems continue to adopt advanced diagnostic capabilities, the procalcitonin market is poised to benefit from these technological advancements.
Market Challenges
High Cost of Diagnostic Kits
Despite the increasing demand for procalcitonin testing, the high cost associated with diagnostic kits remains a significant barrier to market penetration. Reports show that the average price for a procalcitonin diagnostic kit ranges from USD 30 to USD 100, which can be prohibitive for smaller healthcare facilities. Particularly in regions where healthcare budgets are constrained, this high cost can deter adoption. As medical institutions seek cost-cutting measures, this challenge inhibits broader implementations of procalcitonin testing protocols.
Regulatory Constraints
Navigating the complex regulatory landscape is another considerable challenge facing the PCT market. The FDA has stringent guidelines for the approval of diagnostic tests, which can prolong the time to market for new PCT diagnostic solutions. The regulatory approval process can take anywhere from several months to several years, resulting in delayed product launches, according to the FDA’s 2023 updates on regulatory pathways. Furthermore, compliance with clinical performance requirements and post-market surveillance adds an additional layer of difficulty, limiting smaller companies from entering the market effectively.
Opportunities
Growing Awareness of PCT Testing
There is a pronounced increase in healthcare professionals’ awareness regarding the significance of procalcitonin as a biomarker for bacterial infections and sepsis. The content from various clinical guideline publications indicates that more than 70% of healthcare providers are now integrating procalcitonin testing into their practice as a standard diagnostic tool. This shift demonstrates a growing commitment to evidence-based medicine, signifying a positive trajectory for the PCT market. As the medical community recognizes the importance of swift and accurate diagnostics, the uptake of PCT testing is anticipated to escalate in the coming years, enhancing overall patient outcomes.
Integration of PCT Testing in Clinical Protocols
The integration of procalcitonin testing into clinical practice guidelines is becoming increasingly common as hospitals aim to standardize care pathways. Reports from leading health organizations highlight a surge in the number of institutional protocols that now include the assessment of procalcitonin levels for the early diagnosis of sepsis. For instance, hospitals have recorded a marked increase in the adoption rate of PCT testing, showcasing its potential in impacting treatment decisions and antibiotic stewardship. As healthcare providers understand the clinical benefits associated with PCT, such as reducing unnecessary antibiotic use, the market is poised for significant expansion.
Future Outlook
Over the next several years, the USA Procalcitonin market is anticipated to experience significant growth driven by advancements in diagnostic technologies, increasing healthcare expenditure, and a higher emphasis on improving patient outcomes through early diagnostics. Furthermore, the mounting prevalence of sepsis and respiratory infections, alongside the growing awareness among healthcare professionals regarding the benefits of PCT testing, are expected to substantially contribute to the market’s expansion. As healthcare systems evolve to incorporate innovative solutions, the demand for Procalcitonin testing is projected to rise, reflecting positively on market dynamics.
Major Players
- Roche Diagnostics
- Thermo Fisher Scientific
- bioMérieux
- Abbott Laboratories
- Siemens Healthineers
- Ortho Clinical Diagnostics
- Hologic
- Fujifilm
- Danaher Corporation
- PerkinElmer
- QIAGEN
- Mindray
- Becton, Dickinson and Company
- Sysmex Corporation
- Agilent Technologies
Key Target Audience
- Healthcare Institutions (Hospitals and Clinics)
- Clinical Laboratories
- Diagnostic Equipment Manufacturers
- Pharmaceutical Companies
- Investments and Venture Capitalist Firms
- Government and Regulatory Bodies (FDA, CDC)
- Research and Development Organizations
- Healthcare Purchasing Groups
Research Methodology
Step 1: Identification of Key Variables
The initial phase involves constructing an ecosystem map encompassing all major stakeholders within the USA Procalcitonin market. This step is underpinned by extensive desk research, utilizing secondary and proprietary databases to gather comprehensive industry-level information. The primary objective is to identify and define the critical variables that influence market dynamics, including technology advancements, regulatory frameworks, and consumer preferences.
Step 2: Market Analysis and Construction
In this phase, we compile and analyze historical data related to the USA Procalcitonin market. This includes assessing market penetration, the ratio of diagnostic testing to treatment practices, and the resultant revenue generation. Additionally, an evaluation of service quality statistics will be conducted to ensure the reliability and accuracy of the revenue estimates, enabling an informed understanding of the current market structure.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses will be developed and subsequently validated through consultations with industry experts representing a diverse array of companies. These consultations will provide valuable operational and financial insights directly from industry practitioners, thus refining and corroborating the market data collected in earlier phases. Insights from these consultations are critical for understanding the practical implications of market statistics.
Step 4: Research Synthesis and Final Output
The final phase involves direct engagement with multiple diagnostic laboratories and healthcare providers to acquire detailed insights into product segments, sales performance, consumer preferences, and other pertinent factors. This interaction will substantiate and complement the statistics derived from a bottom-up approach, ensuring a comprehensive, accurate, and validated analysis of the USA Procalcitonin market.
- Executive Summary
- Research Methodology
(Market Definitions and Assumptions, Abbreviations, Market Sizing Approach, Consolidated Research Approach, Understanding Market Potential Through In-Depth Industry Interviews, Primary Research Approach, Limitations and Future Conclusions)
- Definition and Scope
- Overview Genesis
- Timeline of Major Players
- Business Cycle
- Supply Chain and Value Chain Analysis
- Growth Drivers
Increasing Incidence of Sepsis
Advances in Diagnostic Technologies
Rising Health Expenditure - Market Challenges
High Cost of Diagnostic Kits
Regulatory Constraints - Opportunities
Growing Awareness of PCT Testing
Integration of PCT Testing in Clinical Protocols - Trends
Shift Towards Rapid Diagnostics
Innovations in Laboratory Testing - Government Regulation
Compliance Standards
Market Access Regulations - SWOT Analysis
- Stakeholder Ecosystem
- Porter’s Five Forces
- By Value, 2019-2024
- By Volume, 2019-2024
- By Average Price, 2019-2024
- By Product Type (In Value %)
Test Kits
Reagents
Instruments - By Application (In Value %)
Sepsis Diagnosis
Critical Care Testing
Respiratory Tract Infections - By End User (In Value %)
Hospitals
Clinical Laboratories
Point of Care Testing - By Distribution Channel (In Value %)
Direct Sales
Distributors
Online Sales - By Region (In Value %)
Northeast
Midwest
South
West
- Market Share of Major Players on the Basis of Value/Volume, 2024
Market Share of Major Players by Type of Procalcitonin (PCT) Segment, 2024 - Cross Comparison Parameters (Company Overview, Business Strategies, Recent Developments, Strength, Weakness, Organizational Structure, Revenues, Number of Touchpoints, Distribution Channels, Number of Dealers and Distributors, Margins, Production Plant, Capacity, Unique Value offering and others)
- SWOT Analysis of Major Players
- Pricing Analysis Basis SKUs for Major Players
- Detailed Profiles of Major Companies
Thermo Fisher Scientific
Roche Diagnostics
bioMérieux
Siemens Healthineers
Abbott Laboratories
Ortho Clinical Diagnostics
Hologic
Fujifilm
Danaher Corporation
PerkinElmer
QIAGEN
Mindray
Becton, Dickinson and Company
Sysmex Corporation
Agilent Technologies
- Market Demand and Utilization
- Purchasing Power and Budget Allocations
- Regulatory and Compliance Requirements
- Needs, Desires, and Pain Point Analysis
- Decision-Making Process
- By Value, 2025-2030
- By Volume, 2025-2030
- By Average Price, 2025-2030


