The healthcare industry operates within one of the most complex and high-stakes supply chain ecosystems in the world. From critical medical devices and pharmaceuticals to hospital consumables and diagnostics, uninterrupted access to quality-assured supplies is vital for patient safety and clinical efficiency. However, recent global disruptions have exposed deep vulnerabilities in healthcare procurement systems.
In this context, procurement research has emerged as a strategic enabler of resilience. By combining supplier intelligence, market analytics, and regulatory insight, healthcare organizations can strengthen sourcing decisions and proactively mitigate risks. Procurement research provides the visibility needed to identify reliable suppliers, ensure compliance, and optimize cost structures, transforming procurement from a reactive function into a core pillar of healthcare continuity and trust.
Why Procurement Research Matters in Clinical Supply Chain Risk Management
In healthcare, supply chain disruptions can have life-threatening consequences. From shortages of essential drugs to delayed delivery of diagnostic equipment, any procurement inefficiency directly impacts patient outcomes and institutional credibility. Procurement research enables healthcare organizations to anticipate, evaluate, and mitigate these risks through a systematic, data-driven approach.
Key reasons procurement research is essential to healthcare risk management include:
- Supplier Reliability and Qualification: Evaluating suppliers’ financial stability, compliance records, and capacity to ensure they meet stringent quality and safety standards.
- Regulatory and Quality Compliance: Tracking adherence to frameworks such as FDA, CE, and WHO-GMP certifications to prevent non-compliant sourcing and potential product recalls.
- Category and Market Intelligence: Monitoring price trends, raw material dependencies, and demand-supply dynamics for pharmaceuticals, devices, and consumables to reduce procurement volatility.
- Counterfeit and Substandard Product Prevention: Leveraging supplier due diligence and traceability audits to minimize exposure to non-genuine or low-quality products entering the clinical supply chain.
- Geopolitical and Logistics Risk Mapping: Analyzing trade dependencies, import restrictions, and transport vulnerabilities to ensure continuity during global disruptions.
Procurement research transforms healthcare sourcing into a strategic risk management function, offering the visibility and intelligence needed to protect patient safety, maintain compliance, and sustain clinical operations even amid uncertainty.
Nexdigm’s Healthcare Procurement Research Framework
Nexdigm’s Healthcare Procurement Research Framework provides a structured, intelligence-driven approach to identifying, evaluating, and managing suppliers across the clinical supply ecosystem. Our framework integrates regulatory compliance, cost analytics, and risk assessment to help healthcare organizations build resilient, transparent, and sustainable procurement operations.
Key pillars of our framework include:
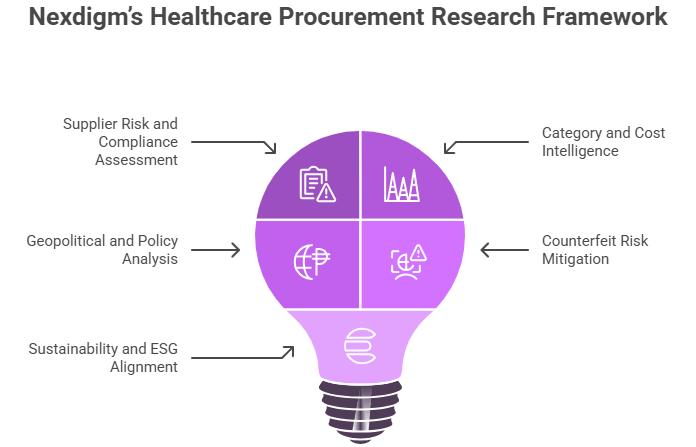
- Supplier Risk and Compliance Assessment: Conducting in-depth evaluation of suppliers’ quality certifications, audit histories, production capabilities, and adherence to regulatory standards such as FDA, CE, and ISO 13485.
- Category and Cost Intelligence: Analyzing category-specific trends for pharmaceuticals, medical devices, and hospital consumables to track input costs, pricing benchmarks, and market availability.
- Geopolitical and Policy Analysis: Monitoring trade restrictions, import dependencies, and healthcare policy changes that can influence sourcing decisions and supplier diversification.
- Counterfeit Risk Mitigation: Leveraging supplier traceability checks, product authentication research, and third-party verification to ensure product integrity.
- Sustainability and ESG Alignment: Assessing suppliers on environmental standards, ethical labor practices, and waste management to promote responsible procurement.
Through this comprehensive framework, Nexdigm enables hospitals, pharmaceutical firms, and med-tech manufacturers to transform procurement into a proactive safeguard, reducing operational vulnerabilities, ensuring uninterrupted supply, and upholding clinical excellence.
To take the next step, simply visit our Request a Consultation page and share your requirements with us.
Harsh Mittal
+91-8422857704

