The medical device industry stands at the crossroads of innovation and global opportunity. Advancements in diagnostics, minimally invasive technologies, and connected healthcare are driving unprecedented demand across Asia, the Middle East, and emerging Europe. Governments are actively investing in healthcare infrastructure, while hospitals and clinics are adopting new technologies to improve care delivery.
For manufacturers, this creates immense potential but also intense complexity. Each market has unique device regulations, approval pathways, reimbursement systems, and distribution challenges. Expanding internationally without a clear go-to-market roadmap often leads to delayed approvals, unviable pricing, or misaligned partnerships.
To scale effectively, MedTech players need structured support that combines market intelligence, regulatory expertise, and financial modeling. Nexdigm’s Medical Device Go-to-Market Entry Consulting Services provide precisely that, helping companies navigate compliance, identify the right partners, and localize commercial strategies to accelerate international growth.
Nexdigm’s Medical Device Go-to-Market Entry Consulting Framework
Successfully expanding a medical device business into new markets demands regulatory precision, local insight, and commercial adaptability. Nexdigm’s Go-to-Market Entry Consulting Framework helps MedTech companies translate innovation into scalable global presence through a structured, data-driven approach.
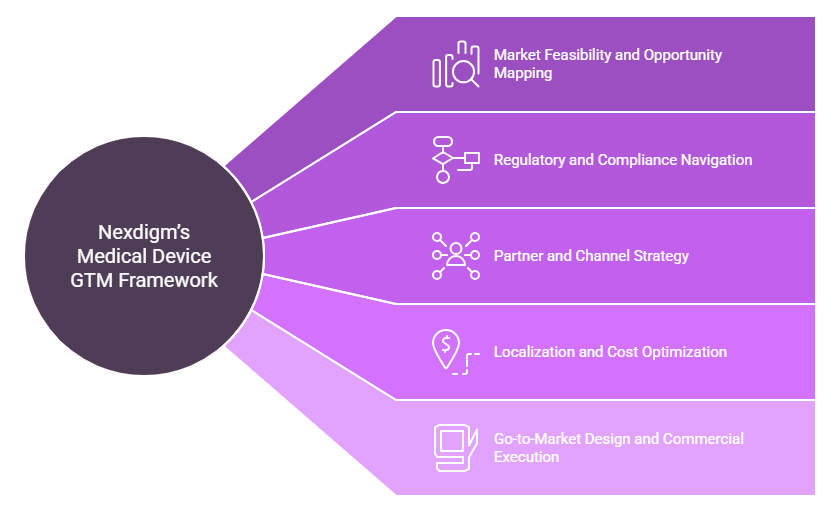
- Market Feasibility and Opportunity Mapping: Nexdigm begins with an in-depth assessment of market potential by evaluating disease burden, infrastructure maturity, and device adoption trends across target regions. Using TAM–SAM–SOM modeling, the team identifies high-demand specialties such as cardiology, orthopedics, and imaging, and quantifies entry potential based on hospital readiness and procurement budgets.
- Regulatory and Compliance Navigation: Each country’s regulatory pathway is distinct. Nexdigm helps clients decode device classification, registration processes, and certification equivalencies, whether under FDA, CE, CDSCO, SFDA, or TGA standards. By aligning documentation and clinical evaluation requirements, the firm minimizes approval delays and ensures products meet both regulatory and post-market surveillance expectations.
- Partner and Channel Strategy: Selecting the right distributors or hospital procurement networks can determine long-term success. Nexdigm identifies and validates channel partners, group purchasing organizations (GPOs), and logistics providers through detailed due diligence covering financial health, compliance history, and distribution capacity. This ensures that every partnership enhances operational reach and market credibility.
- Localization and Cost Optimization: To strengthen competitiveness, Nexdigm evaluates opportunities for local assembly, packaging, or warehousing under regional incentive programs such as PLI (India) or GCC manufacturing zones. Comparative cost modeling and supply-chain mapping allow clients to balance cost efficiency with compliance requirements while maintaining quality standards.
- Go-to-Market Design and Commercial Execution: The final phase translates strategy into action. Nexdigm develops pricing, reimbursement, and sales channel blueprints tailored to local healthcare structures. Financial models help clients plan resource allocation efficiently. This integrated design enables faster market penetration and sustainable long-term presence.
With this holistic framework, Nexdigm transforms complex market entry into a streamlined, insight-led growth process for global medical device firms.
How Go-to-Market Consulting Fuels Global Competitiveness
In the MedTech sector, innovation alone isn’t enough, success depends on how effectively companies commercialize across borders.
- Faster Market Access: Structured go-to-market planning helps firms navigate approvals, secure partnerships, and launch products faster while staying compliant.
- Smarter Capital Deployment: By prioritizing high-return, policy-friendly markets, Nexdigm ensures expansion investments deliver measurable results.
- Local Credibility and Scalability: Tailored localization, transparent pricing, and vetted partners strengthen brand trust and create a foundation for long-term growth.
With the right go-to-market strategy, medical device firms can turn expansion into a competitive advantage, entering global markets faster, leaner, and stronger.
At Nexdigm, our Medical Device Go-to-Market Entry Consulting Services empower manufacturers and healthcare technology firms to commercialize globally with confidence. We combine market intelligence, regulatory expertise, and financial modeling to help you identify the right markets, build trusted local partnerships, and scale efficiently.
To take the next step, simply visit our Request a Consultation page and share your requirements with us.
Harsh Mittal
+91-8422857704

