The medical devices industry is at the forefront of healthcare transformation, driven by technological innovation, digital integration, and rising global demand. From advanced diagnostic tools and wearables to minimally invasive surgical devices, the sector is expanding and the road to success is becoming increasingly complex.
Two of the most pressing challenges for manufacturers today are achieving the right product–market fit and navigating diverse regulatory frameworks across geographies. A device that is clinically effective but misaligned with market needs or delayed by lengthy approvals risks falling behind competitors.
This is where strategic benchmarking plays a vital role. By comparing performance, regulatory efficiency, and adoption models against global leaders, medical device companies can accelerate their journey to market, optimize compliance processes, and ensure their products meet both clinical expectations and commercial realities.
Key Insights Driving Medical Device Competitiveness
Global benchmarking highlights how medical device companies are reshaping strategies to achieve faster approvals, stronger adoption, and long-term competitiveness. Some of the most critical insights include:
- Early Regulatory Alignment: Global leaders integrate regulatory requirements during the R&D phase, significantly reducing approval timelines across markets such as the U.S. (FDA), Europe (MDR), and Asia. This proactive approach ensures faster time-to-market and minimizes costly rework.
- Patient-Centric Design: Benchmarking shows that devices tailored to usability, accessibility, and affordability enjoy higher adoption rates. Companies prioritizing human-centered design are outperforming peers in both mature and emerging markets.
- Digital Integration & IoMT: The fastest-growing players are embedding digital capabilities into devices, setting new benchmarks for connected care and remote monitoring.
- Sustainability & ESG Standards: Leaders are adopting eco-friendly manufacturing and circular economy practices. Benchmarking reveals that ESG compliance is increasingly tied to regulatory approval and market trust.
- Post-Market Vigilance: Effective post-launch monitoring, including safety reporting and performance tracking, has become a critical competitive differentiator, influencing both reputation and regulatory standing.
These insights confirm that competitiveness in the medical devices industry is no longer driven by innovation alone but by a holistic balance of speed, compliance, usability, and sustainability.
Nexdigm’s Medical Devices Strategic Benchmarking Approach
At Nexdigm, we help medical device companies accelerate both market readiness and regulatory efficiency by applying a structured benchmarking methodology. Our medical devices strategic benchmarking approach is designed to identify best practices, uncover inefficiencies, and deliver actionable insights that strengthen competitiveness in a highly regulated industry.
Key elements of our benchmarking framework include:
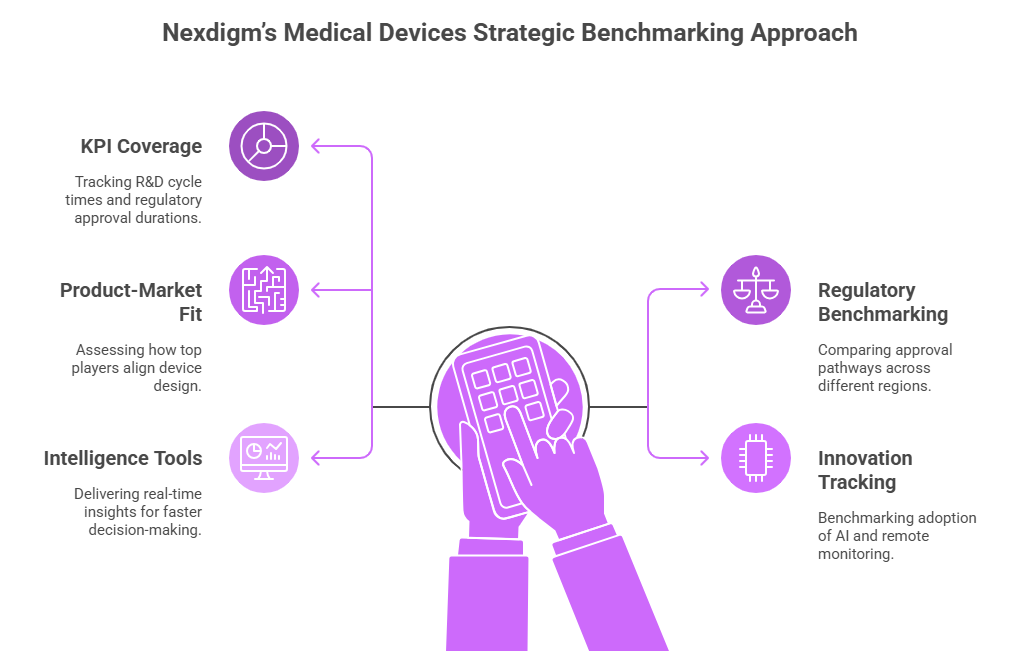
- Comprehensive KPI Coverage: Tracking R&D cycle times, regulatory approval durations, clinical validation efficiency, and post-market surveillance effectiveness.
- Cross-Market Regulatory Benchmarking: Comparing approval pathways across the U.S., Europe, and Asia to highlight acceleration opportunities and compliance best practices.
- Competitor Product-Market Fit Analysis: Assessing how top players align device design, pricing, and positioning with unmet clinical and commercial needs.
- Innovation & Digital Integration Tracking: Benchmarking adoption of AI, IoMT, and remote monitoring technologies to measure their role in driving adoption and differentiation.
- Actionable Dashboards & Intelligence Tools: Delivering real-time insights into competitor launches, regulatory updates, and global adoption trends for faster decision-making.
Through this structured approach, Nexdigm enables medical device companies to optimize product development, reduce regulatory bottlenecks, and improve market success rates, ensuring sustained competitiveness in a dynamic healthcare ecosystem.
To take the next step, simply visit our Request a Consultation page and share your requirements with us.
Harsh Mittal
+91-8422857704

