The MedTech industry stands at the intersection of healthcare innovation and patient safety. From surgical instruments and diagnostic equipment to implantable devices and digital health solutions, every component sourced plays a direct role in clinical outcomes. Yet, as medical technology evolves, procurement teams face increasing complexity, balancing innovation, compliance, and cost efficiency across global supplier networks.
Traditional sourcing methods are no longer sufficient in a market defined by stringent regulations, fluctuating input costs, and fast-paced product development cycles. This is where procurement research and supplier evaluation become indispensable. By integrating supplier intelligence, quality assessments, and compliance insights, MedTech companies can identify trusted partners, reduce operational risks, and ensure that procurement decisions support both technological advancement and patient safety.
Why Supplier Evaluation and Research are Critical in MedTech Procurement
In the MedTech ecosystem, where precision, quality, and compliance directly impact patient outcomes, supplier evaluation is a strategic imperative. The sourcing of medical devices, diagnostic components, and digital health technologies involves navigating complex supplier landscapes, evolving regulatory standards, and escalating production costs. Procurement research enables MedTech companies to make informed, risk-aware decisions while maintaining agility and innovation.
Key reasons supplier evaluation and research are vital in MedTech procurement include:
- Regulatory Compliance Assurance: Ensures that suppliers meet international standards such as FDA, CE, and ISO 13485, safeguarding against compliance lapses and costly recalls.
- Quality and Traceability: Evaluates suppliers’ manufacturing precision, sterilization processes, and documentation systems to maintain consistent product quality and traceability.
- Innovation and R&D Readiness: Identifies suppliers with the capability to co-develop and scale next-generation devices, sensors, and diagnostic solutions.
- Cost and Lifecycle Optimization: Provides visibility into total cost of ownership (TCO) by analyzing raw material, production, logistics, and maintenance costs.
- Supply Continuity and Risk Management: Monitors supplier financial stability, capacity utilization, and geopolitical exposure to prevent disruptions in critical device supply.
Through structured supplier evaluation and procurement research, MedTech organizations can align sourcing decisions with innovation roadmaps, ensuring resilience, compliance, and clinical excellence in every link of the supply chain.
Nexdigm’s MedTech Procurement Research Framework
Nexdigm’s MedTech Procurement Research Framework is designed to help healthcare and medical technology organizations make data-driven, compliant, and innovation-aligned sourcing decisions. By combining supplier analytics, category insights, and cost intelligence, our framework enables procurement teams to strengthen visibility, mitigate risks, and accelerate product development cycles.
Core pillars of our framework include:
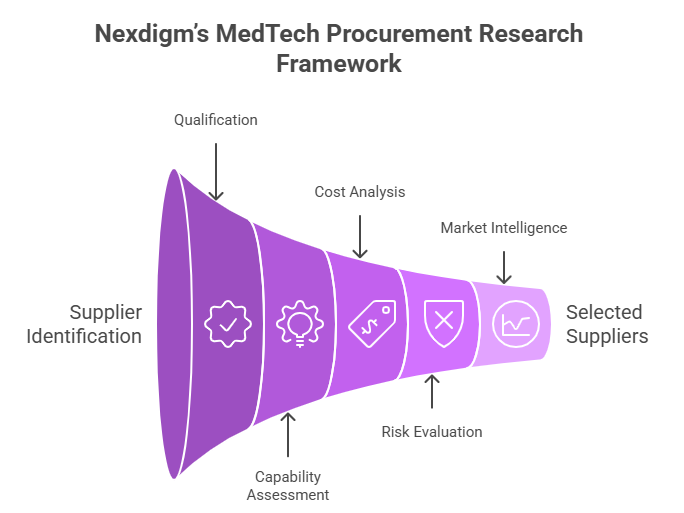
- Supplier Identification and Qualification: Mapping global and regional suppliers based on production specialization, certifications (FDA, CE, ISO 13485), and scalability for emerging MedTech technologies.
- Capability and Innovation Assessment: Evaluating suppliers on R&D investment, engineering expertise, digital integration, and readiness for next-generation product collaboration.
- Cost and Total Cost of Ownership (TCO) Analysis: Analyzing end-to-end cost drivers to ensure value-based procurement.
- Risk and Compliance Evaluation: Assessing suppliers’ financial health, supply stability, ESG adherence, and track record in regulatory audits to minimize operational and reputational risks.
- Category and Market Intelligence: Tracking price fluctuations, technology shifts, and competitive benchmarks across device categories such as diagnostics, implants, and therapeutic equipment.
By integrating these elements, Nexdigm’s procurement research empowers MedTech companies to build high-performing, compliant, and innovation-driven supplier ecosystems that elevate both operational efficiency and clinical value.
To take the next step, simply visit our Request a Consultation page and share your requirements with us.
Harsh Mittal
+91-8422857704

