Market Overview
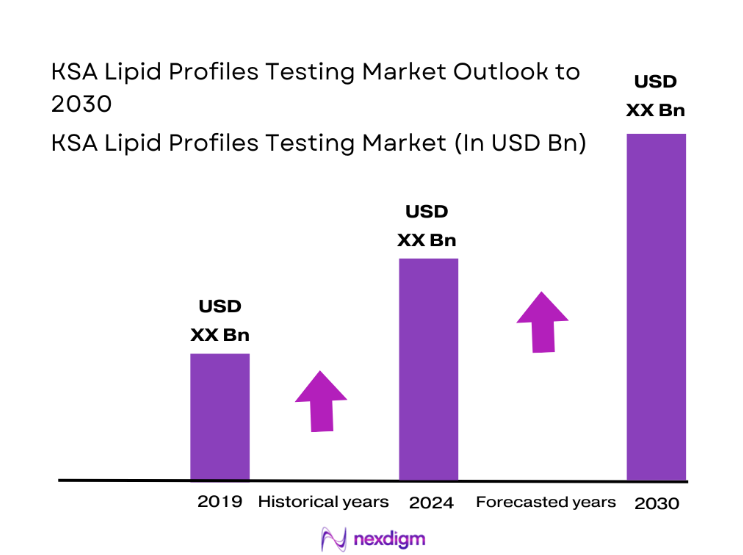
The KSA lipid profiles testing market is valued at USD ~, reflecting its role as a foundational diagnostic service within the national healthcare ecosystem. Demand is structurally driven by the rising clinical focus on cardiovascular risk stratification, long-term diabetes management, and preventive screening mandates across public and private care settings. Lipid testing has become embedded in routine care pathways, from primary clinics to tertiary hospitals, making it a high-frequency, high-volume diagnostic category that underpins chronic disease programs, insurance-backed health packages, and corporate wellness initiatives across the healthcare value chain.
Within the country, demand is concentrated in Riyadh, Jeddah, and Dammam due to their dense clusters of tertiary hospitals, reference laboratories, and corporate healthcare providers. Riyadh dominates through centralized government healthcare operations and national specialty institutes, while Jeddah benefits from its strong private hospital ecosystem and medical tourism activity. Dammam is driven by occupational health demand from industrial sectors. On the supply side, the market is shaped by global diagnostics technology leaders and multinational manufacturing hubs that influence analyzer standards, reagent innovation, and automation frameworks, setting the performance benchmarks adopted by laboratories nationwide.
Market Segmentation
By Test Type
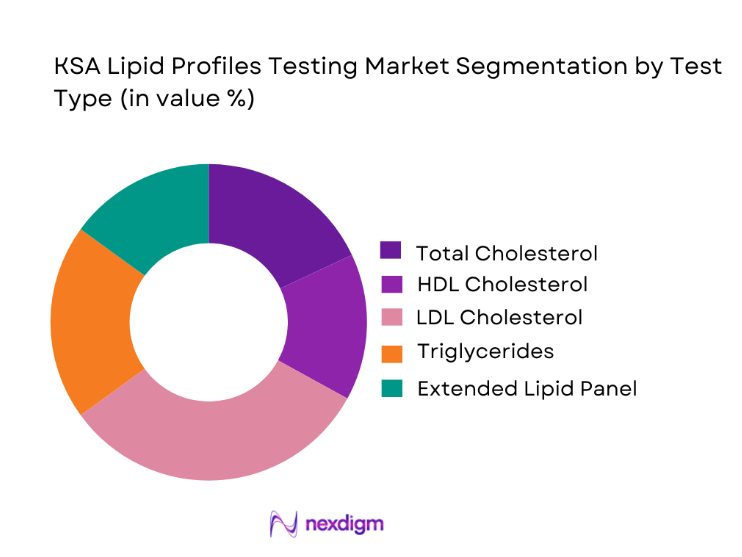
The KSA lipid profiles testing market by test type is led by LDL cholesterol testing, which has emerged as the dominant sub-segment due to its central role in cardiovascular risk management and therapeutic decision-making. Clinicians increasingly rely on LDL values to guide statin initiation, dosage adjustments, and long-term patient monitoring, making it the most clinically actionable lipid parameter. National guidelines emphasizing early detection of dyslipidemia and aggressive management of high-risk populations have further elevated the importance of LDL testing. In addition, the shift from calculated to direct LDL assays in complex cases has increased both test frequency and value realization. As preventive cardiology gains momentum and chronic disease management programs expand, LDL testing continues to anchor lipid profile demand across hospitals, diagnostic laboratories, and primary care networks.
By End-Use
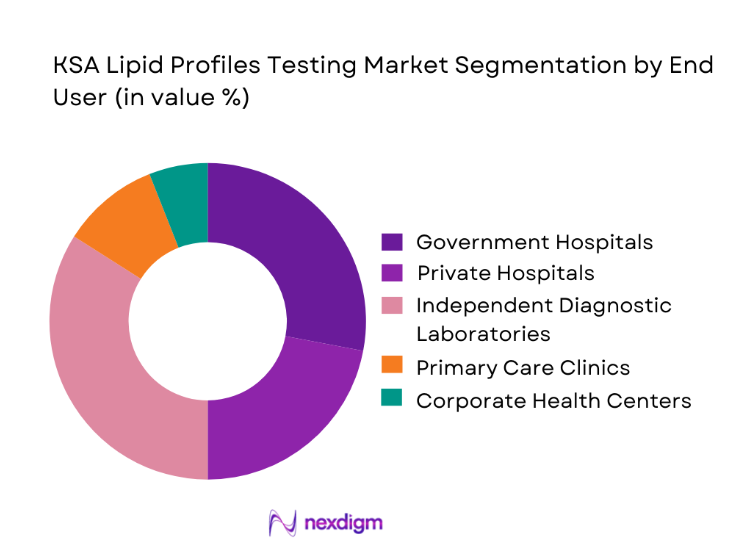
Among end-use customer types, independent diagnostic laboratories dominate the KSA lipid profiles testing market due to their expanding footprint, operational efficiency, and central role in outsourced diagnostics. These laboratories have invested heavily in high-throughput analyzers, automation lines, and digital reporting systems, enabling them to process large volumes of lipid tests at competitive cost structures. Their strong positioning in preventive health packages and corporate wellness programs has further amplified routine lipid testing demand outside hospital settings. Additionally, partnerships with insurance providers and government screening initiatives have increased test inflow consistency. As healthcare delivery shifts toward decentralized and patient-centric models, independent laboratories continue to strengthen their leadership by offering faster turnaround times, broader geographic access, and integrated home collection services that support sustained growth in lipid profile testing volumes.
Competitive Landscape
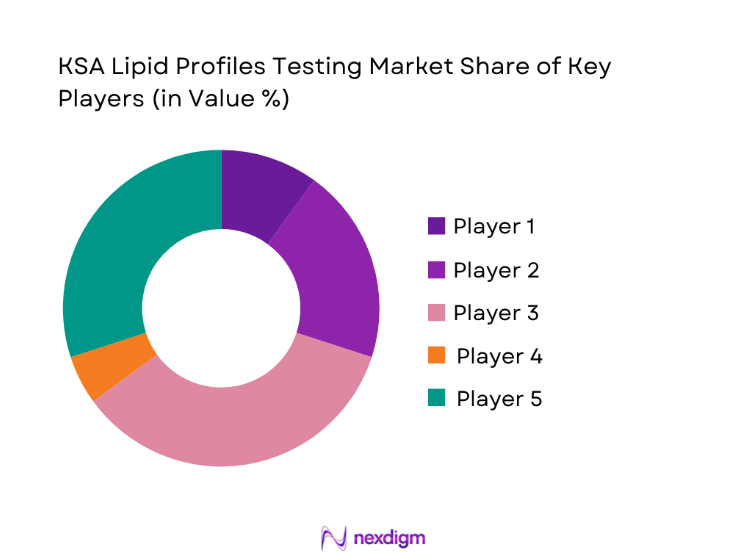
The KSA Lipid Profiles Testing market is dominated by a few major players, including Roche Diagnostics and global or regional brands like Abbott Diagnostics, Siemens Healthineers, and Beckman Coulter. This consolidation highlights the significant influence of these key companies.
| Company | Establishment Year | Headquarters | Installed Base in KSA | Reagent Rental Penetration | SFDA Registered Products | Local Service Network | Key End-User Focus | Technology Strength |
| Roche Diagnostics | 1896 | Switzerland | ~ | ~ | ~ | ~ | ~ | ~ |
| Abbott Diagnostics | 1888 | USA | ~ | ~ | ~ | ~ | ~ | ~ |
| Siemens Healthineers | 1847 | Germany | ~ | ~ | ~ | ~ | ~ | ~ |
| Beckman Coulter | 1935 | USA | ~ | ~ | ~ | ~ | ~ | ~ |
| Mindray Medical | 1991 | China | ~ | ~ | ~ | ~ | ~ | ~ |
KSA Lipid Profiles Testing Market Analysis
Growth Drivers
Rising cardiovascular disease burden
The increasing prevalence of heart disease and metabolic disorders is fundamentally reshaping diagnostic priorities across the healthcare system. As clinicians focus on early risk identification and long-term disease management, lipid profiling has become a standard component of routine patient assessment. This shift drives consistent test volumes across hospitals and clinics while also strengthening demand from preventive health programs. The outcome is a structurally resilient market where lipid testing is no longer episodic but embedded in continuous care pathways.
Expansion of preventive screening programs
Government-led and private sector preventive health initiatives are expanding the scope of routine diagnostics beyond symptomatic care. Large-scale screening programs targeting high-risk populations have elevated lipid profiling as a first-line diagnostic tool. This approach increases testing frequency among asymptomatic individuals, broadening the market base and reinforcing the role of diagnostics in population health management.
Challenges
Pricing pressure from centralized procurement
Centralized purchasing frameworks have intensified competition among suppliers, leading to sustained pressure on reagent pricing and service margins. While this improves cost efficiency for healthcare providers, it constrains revenue growth for diagnostics companies and limits their flexibility in investing in innovation and localized support infrastructure. Over time, this dynamic may affect service quality and technology refresh cycles across laboratories.
Shortage of specialized laboratory workforce
The rapid expansion of diagnostic services has outpaced the availability of trained laboratory professionals, particularly in secondary cities and remote regions. Workforce gaps affect turnaround times, quality assurance, and the adoption of advanced automation platforms. Without parallel investments in training and professional development, operational bottlenecks may limit the full growth potential of lipid testing services nationwide.
Opportunities
Automation-led laboratory efficiency gains
The adoption of fully automated analyzers and integrated laboratory lines presents a major opportunity to enhance throughput, reduce manual errors, and optimize cost per test. As laboratories scale operations, automation enables them to handle rising lipid test volumes without proportional increases in staffing, improving both profitability and service consistency. This technological shift also supports faster reporting, which is critical for time-sensitive clinical decision-making.
Expansion of decentralized testing models
Growing demand for patient convenience is accelerating the adoption of decentralized diagnostics, including community-based laboratories and home collection services. Lipid profiling, being a high-frequency and low-complexity test category, is well suited to this model. Expanding access beyond hospital settings opens new revenue streams while improving early detection rates across underserved populations.
Future Outlook
The KSA lipid profiles testing market is positioned for steady expansion as preventive healthcare becomes a central pillar of national health strategy. Continued investment in laboratory automation, digital health integration, and decentralized service delivery will enhance accessibility and operational efficiency. At the same time, the alignment of insurance coverage with routine diagnostics and the growth of corporate wellness programs will sustain long-term demand, reinforcing lipid testing as a core component of chronic disease management and population health initiatives.
Major Players
- Roche Diagnostics
- Abbott Diagnostics
- Siemens Healthineers
- Beckman Coulter
- Thermo Fisher Scientific
- Randox Laboratories
- Ortho Clinical Diagnostics
- Mindray Medical
- Bio-Rad Laboratories
- DiaSys Diagnostic Systems
- Sekisui Diagnostics
- ARKRAY
- Sysmex Corporation
- Fujifilm Healthcare
- Elitech Group
Key Target Audience
- Hospital groups and healthcare networks
- Independent diagnostic laboratory chains
- Corporate healthcare and occupational health providers
- Medical device and diagnostics distributors
- Investments and venture capitalist firms
- Government and regulatory bodies
- Health insurance companies and third-party administrators
- Healthcare infrastructure developers and hospital project investors
Research Methodology
Step 1: Identification of Key Variables
The research began with mapping the diagnostic ecosystem across public and private healthcare segments to identify key demand and supply variables. Secondary data from procurement systems, hospital utilization records, and diagnostics company disclosures were analyzed to define market boundaries and revenue drivers. This phase established the foundational assumptions guiding the study.
Step 2: Market Analysis and Construction
Historical testing volumes and revenue flows were assessed using a bottom-up approach, aggregating data from laboratory networks and supplier sales channels. Market structure was validated through analysis of service delivery models and pricing frameworks to ensure realistic representation of competitive dynamics.
Step 3: Hypothesis Validation and Expert Consultation
Preliminary market insights were validated through structured discussions with laboratory directors, procurement leaders, and distributor executives. These consultations refined assumptions around technology adoption, purchasing behavior, and service-level expectations, strengthening the credibility of the market model.
Step 4: Research Synthesis and Final Output
The final phase integrated qualitative insights with quantitative modeling to produce a coherent market narrative. Cross-verification across multiple data streams ensured consistency, while expert feedback helped align findings with operational realities in the diagnostics landscape.
- Executive Summary
- Research Methodology (Market definitions and scope boundaries, terminology and abbreviations, lipid testing taxonomy and care pathway mapping, market sizing logic by test volume and analyzer installed base, revenue attribution across reagents calibrators controls and service, primary interview program with hospitals labs distributors and payers, data triangulation and validation approach, assumptions limitations and data gaps)
- Definition and Scope
- Market Genesis and Evolution of Lipid Testing in KSA
- Cardiometabolic Burden and Preventive Screening Drivers
- Care Pathway Mapping Across Primary Care Hospitals and Diagnostic Centers
- Public Procurement and Private Lab Network Dynamics
- Import Dependence and Distributor Managed Supply Ecosystem
- Growth Drivers
High prevalence of obesity diabetes and cardiovascular risk factors
Expansion of preventive screening and chronic disease programs
Growth of private diagnostics and lab network capacity
Greater focus on early risk stratification and treatment optimization
Employer led screening initiatives and wellness programs - Challenges
Tender driven pricing compression for routine chemistry reagents
Preanalytical variability and fasting compliance issues
Limited reimbursement coverage for advanced lipid biomarkers
Standardization gaps across decentralized testing sites
Supply continuity risk for imported reagents and calibrators - Opportunities
Advanced lipid testing growth for high risk patient stratification
POCT lipid testing expansion in primary care and remote sites
Bundled reagent and analyzer contracting models for lab networks
Integration of lipid results into cardiometabolic registries and analytics
Clinical education programs for familial hypercholesterolemia screening - Trends
Shift toward non fasting lipid testing protocols in routine care
Growing adoption of lipoprotein a and apolipoprotein testing
Greater use of reflex testing for high risk lipid abnormalities
Expansion of automated lab lines and higher throughput platforms
Increasing emphasis on QC analytics and accreditation readiness - Regulatory & Policy Landscape
- SWOT Analysis
- Stakeholder & Ecosystem Analysis
- Porter’s Five Forces Analysis
- Competitive Intensity & Ecosystem Mapping
- By Value, 2019–2024
- By Test Volume, 2019–2024
- By Hospital vs Independent Lab Revenue Split, 2019–2024
- By Routine Lipid Panel vs Advanced Lipid Testing Split, 2019–2024
- By Fleet Type (in Value %)
Government hospitals and medical cities
Primary healthcare centers
Private hospital networks
Independent diagnostic laboratories
Corporate and occupational health providers - By Application (in Value %)
Cardiovascular risk screening and prevention
Diabetes and metabolic syndrome monitoring
Statin therapy initiation and follow up monitoring
Pediatric lipid screening and familial hypercholesterolemia workup
Preoperative and inpatient risk assessment testing - By Technology Architecture (in Value %)
Automated clinical chemistry analyzers and reagent systems
Point of care lipid testing devices
Direct LDL and non HDL testing workflows
Apolipoprotein and lipoprotein a testing platforms
Advanced lipid particle analysis and specialty assays - By Connectivity Type (in Value %)
Standalone analyzers with local reporting
LIS integrated laboratory workflows
EHR integrated primary care ordering pathways
Cloud enabled QC analytics and inventory management
Remote service monitoring and uptime support - By End-Use Industry (in Value %)
Clinical laboratories and pathology networks
Primary care and family medicine clinics
Cardiology and endocrinology specialty centers
Hospitals inpatient care programs
Public health screening and wellness providers - By Region (in Value %)
Riyadh Region
Makkah Region
Eastern Province
Madinah Region
Asir and Southern Regions
- Competitive ecosystem structure across clinical chemistry majors specialty assay providers and distributors
- Positioning driven by throughput assay performance and service footprint
- Partnership models between OEMs hospital groups and lab networks
- Cross Comparison Parameters (analytical accuracy and traceability alignment, throughput capacity and automation fit, direct LDL assay performance, reagent stability and storage requirements, LIS and EHR connectivity readiness, QC and calibration burden, advanced lipid menu breadth, cost per reportable result)
- SWOT analysis of major players
- Pricing and commercial model benchmarking
- Detailed Profiles of Major Companies
Roche Diagnostics
Abbott
Siemens Healthineers
Beckman Coulter
Ortho Clinical Diagnostics
Mindray
Horiba Medical
Thermo Fisher Scientific
DiaSorin
Randox Laboratories
Sekisui Diagnostics
Sysmex
QuidelOrtho
Bio Rad Laboratories
BD
- Primary care ordering behavior and screening pathway design
- Lab director priorities for throughput reagent stability and QC burden
- Procurement models in public hospitals and private networks
- Decision criteria for central lab versus POCT deployments
- Total cost of ownership drivers across reagents service and uptime
- By Value, 2025–2030
- By Test Volume, 2025–2030
- By Hospital vs Independent Lab Revenue Split, 2025–2030
- By Routine Lipid Panel vs Advanced Lipid Testing Split, 2025–2030


