Market Overview
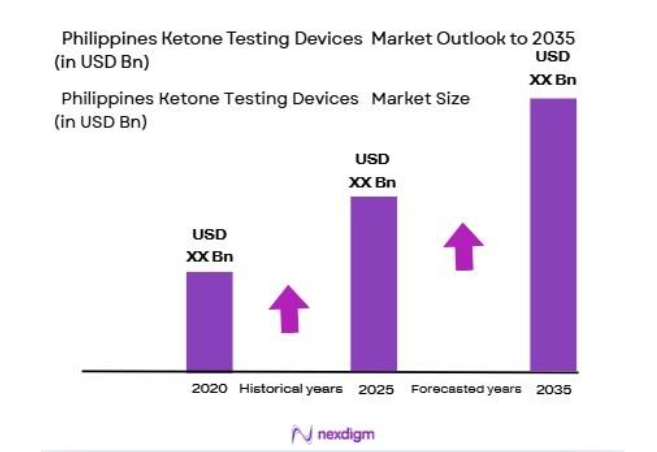
The Philippines Ketone Testing Devices Market is valued at USA ~million in 2025. This market is driven by the increasing prevalence of diabetes and rising health-consciousness among the population. As the number of individuals managing diabetes increases, the demand for effective monitoring tools, including ketone testing devices, has surged. Moreover, government initiatives aimed at improving diabetes management and preventive healthcare have bolstered the adoption of these devices. As of 2024, approximately ~million people in the Philippines are diagnosed with diabetes, further fueling the need for regular ketone testing.
Metro Manila, Cebu, and Davao are the dominant regions for the Ketone Testing Devices market in the Philippines. These urban areas house a significant portion of the population suffering from diabetes, which drives the demand for diagnostic and monitoring tools. Metro Manila, in particular, has the highest concentration of healthcare providers and access to modern medical technologies, making it the key market for ketone testing devices. The population’s increased focus on self-care and diabetes management contributes to these regions’ dominance in the market.
Market Segmentation
By Product Type
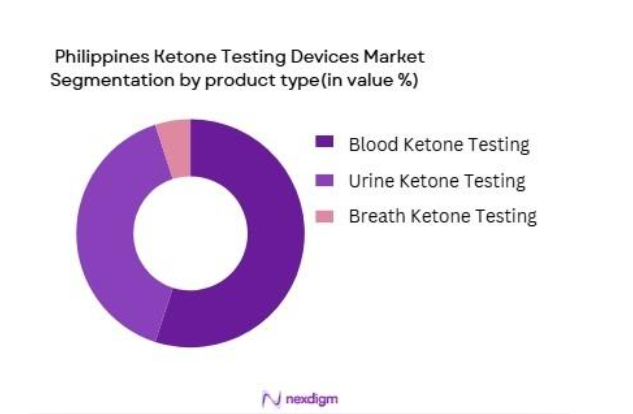
The Philippines Ketone Testing Devices market is segmented by product type into blood ketone testing devices, urine ketone testing devices, and breath ketone testing devices. Blood ketone testing devices dominate this segment, driven by their accuracy and quick results, which are essential for managing ketosis, particularly in diabetic patients. As of 2024, blood ketone meters are widely used in hospitals, clinics, and home care settings due to their reliability. The advancement of blood ketone testing technology, combined with a growing preference for precise results, contributes to the dominance of this sub-segment.
By End-User
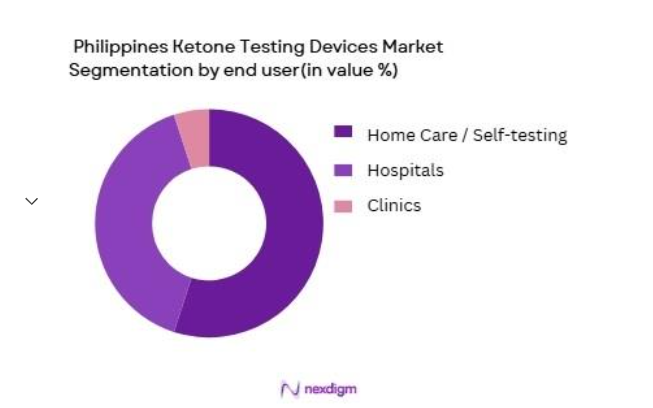
The market is also segmented by end-user into hospitals, clinics, home care, and others. Home care and self-testing have the largest market share due to the increasing trend of individuals managing their diabetes at home. In 2024, the number of diabetic patients opting for home-based ketone monitoring has grown substantially, with home care kits being more affordable and easier to use. This trend reflects the growing preference for personalized health management and the availability of convenient home-use testing devices.
Competitive Landscape
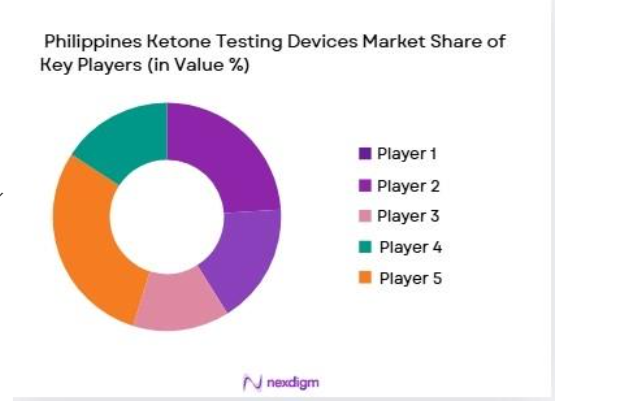
The Philippines Ketone Testing Devices market is dominated by a few key players, with both global and local manufacturers offering products to meet the increasing demand for diabetic care. Leading players in this market include Abbott Laboratories, Roche Diagnostics, and Medtronic. These companies play a significant role due to their established presence in the healthcare market, extensive distribution networks, and continuous innovation in monitoring technologies. Abbott Laboratories, for instance, is well-known for its advanced blood ketone meters, which have seen increasing adoption among patients and healthcare providers in the Philippines.
| Company | Establishment Year | Headquarters | Product Portfolio | Revenue | Key Product Lines | Regional Presence |
| Abbott Laboratories | 1888 | USA | ~ | ~ | ~ | ~ |
| Roche Diagnostics | 1896 | Switzerland | ~ | ~ | ~ | ~ |
| Medtronic | 1949 | USA | ~ | ~ | ~ | ~ |
| Bayer AG | 1863 | Germany | ~ | ~ | ~ | ~ |
| LifeScan (Johnson & Johnson) | 1981 | USA | ~ | ~ | ~ | ~ |
Air Quality Monitoring System Market Analysis
Growth Drivers
Urbanization
Urbanization in Indonesia is a key driver of the air quality monitoring systems market, as the country’s urban population has exceeded ~ million in 2024, particularly concentrated in metropolitan areas like Jakarta, Surabaya, and Bandung. The rapid expansion of cities has led to higher pollution levels due to increased traffic, industrial activities, and construction. In 2024, Jakarta alone has been ranked as one of the most polluted cities globally, with an average annual PM2.5 level of~ µg/m³, far exceeding WHO guidelines. These environmental concerns lead to increased demand for advanced air quality monitoring systems to assess and manage urban pollution
Industrialization
Indonesia’s industrial output has been a significant factor in the rising air pollution, driving the demand for air quality monitoring systems. As of 2024, Indonesia’s industrial sector contributes approximately USD ~ billion to the national GDP, with manufacturing, energy, and mining sectors being major polluters. Areas like East Java and Sumatra, where factories and mining activities are concentrated, have seen drastic increases in pollutants like NOx and SO2. These industrial zones, which account for a large portion of Indonesia’s pollution, are key targets for the deployment of air quality monitoring devices.
Restraints
High Initial Costs
One of the significant barriers to the widespread adoption of air quality monitoring systems in Indonesia is the high initial cost. The installation of advanced air quality monitoring stations, which can cost over USD~per unit, limits the accessibility of these systems to urban municipalities and large industrial sectors. For instance, the capital city of Jakarta has invested heavily in monitoring systems, but other areas still lack sufficient infrastructure. In 2024, the high costs are preventing smaller regions and rural areas from adopting these systems, hindering nationwide environmental management.
Technical Challenges
Technical challenges in the installation and maintenance of air quality monitoring systems have hindered their effectiveness. Indonesia’s diverse geography, combined with unpredictable weather conditions, complicates the calibration, installation, and maintenance of monitoring devices. In 2024, this has been evident in remote regions, where network connectivity issues and lack of technical infrastructure affect the reliability of data collection. These technical challenges impact the accuracy of air quality data, which is crucial for formulating effective policies and interventions to manage pollution levels. Source: Indonesian Environmental Protection Agency, 2024
Opportunities
Technological Advancements
Technological advancements present significant opportunities for the air quality monitoring systems market in Indonesia. The development of low-cost, accurate sensors and portable monitoring units is helping to overcome the high-cost barrier associated with traditional air quality monitoring systems. In 2024, the growing adoption of IoT-enabled devices is enabling real-time air quality monitoring, enhancing the efficiency of pollution management. Furthermore, integration with cloud computing platforms allows for the aggregation of data from various sources, providing more comprehensive air quality insights and enabling better decision-making.
International Collaborations
International collaborations are opening new doors for the growth of air quality monitoring systems in Indonesia. Partnerships with international organizations such as the World Bank and other global environmental agencies are helping Indonesia to improve its monitoring infrastructure. In 2024, these collaborations have provided funding and technological expertise, allowing for the implementation of more sophisticated air quality monitoring systems. The sharing of global best practices and technologies is expected to continue contributing to the market’s expansion as Indonesia works towards improving its air quality management.
Future Outlook
Over the next ~years, the Philippines Ketone Testing Devices market is expected to continue its growth trajectory, driven by the increasing incidence of diabetes and rising healthcare awareness. With advancements in home testing technologies, including mobile applications that sync with ketone meters, the demand for these devices is expected to surge, especially in urban areas. Furthermore, government policies supporting better diabetes management, such as increased insurance coverage for diabetes care, will contribute to the widespread adoption of ketone testing devices. The market is also expected to see innovations in non-invasive testing technologies, further expanding its reach in home healthcare.
Major Players in the Market
- Abbott Laboratories
- Roche Diagnostics
- Medtronic
- Bayer AG
- LifeScan (Johnson & Johnson)
- Ascensia Diabetes Care
- Trividia Health
- ACON Laboratories
- Dexcom
- Nova Biomedical
- Insulet Corporation
- Arkray
- Ketonix
- Genteel
- Xhale
Key Target Audience
- Investments and Venture Capitalist Firms
- Government and Regulatory Bodies
- Hospitals and Healthcare Networks
- Diabetes Management Clinics
- Pharmaceutical Companies
- Retailers and Distributors of Medical Devices
- Medical Insurance Providers
- Self-Care and Home Monitoring Consumers
Research Methodology
Step 1: Identification of Key Variables
In this step, the market landscape of the Philippines Ketone Testing Devices market is mapped out, identifying key factors such as the prevalence of diabetes, the role of government healthcare policies, and technological innovations in ketone testing. A combination of secondary research and market reports will be used to determine the primary drivers and challenges.
Step 2: Market Analysis and Construction
The next phase involves the collection and analysis of historical and current data on the adoption of ketone testing devices. Key parameters such as market size, growth rates, and the contribution of each market segment are examined. We will evaluate the volume of devices sold, end-user behavior, and overall market trends.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses and assumptions are validated through consultations with industry experts, including healthcare providers, device manufacturers, and government officials. Expert interviews help refine data accuracy and gather operational insights to support the market’s growth outlook.
Step 4: Research Synthesis and Final Output
The final phase involves synthesizing data from the previous steps and creating the comprehensive market report. Detailed insights into market segmentation, competitive landscape, and future outlook are incorporated to provide a clear understanding of market trends, challenges, and opportunities.
- Executive Summary
- Research Methodology (Market Definitions and Assumptions, Abbreviations, Market Sizing Approach, Consolidated Research Approach, Understanding Market Potential Through In-Depth Industry Interviews, Primary Research Approach, Limitations and Future Conclusions)
- Definition and Scope
- Market Overview Genesis
- Market Trends & Evolution
- Business Cycle
- Supply Chain and Value Chain Analysis
- Growth Drivers
Increasing Diabetes Prevalence
Growing Health Consciousness and Preventive Care
Rising Adoption of Home Care Devices
- Market Challenges
High Device Costs and Accessibility
Regulatory Hurdles
- Opportunities
Expanding Health Insurance Coverage
Innovations in Ketone Testing Technologies
- Trends
Rising Demand for Non-Invasive Testing Devices
Competition from Alternative Testing Methods
Economic Factors
Regulatory and Compliance Challenges
Counterfeit Products and Substandard Device
- Government Regulation
Regulatory Authority
Device Classification and Registration Requirements
License to Operate
Registration Process and Documentation
- SWOT Analysis
- Porter’s Five Forces
- By Value,2026-2030
- By Volume,2026-2030
- By Average Price,2026-2030
- By Product Type (In Value %)
Blood Ketone Testing Devices
Urine Ketone Testing Devices
Breath Ketone Testing Devices - By End-user (In Value %)
Hospitals and Healthcare Providers
Home Care and Self-Testing
Clinics and Medical Centers
- By Technology (In Value %)
Electrochemical Sensors
Colorimetric Sensors
Optical Sensors
Visayas
Mindanao
- Market Share of Major Players
- Cross Comparison Parameters (Company Overview, Business Strategies, Recent Developments, Strengths, Weaknesses, Organizational Structure, Revenues, Revenues by Product Type, Number of Distribution Channels, Number of Dealers and Distributors, Margins, Production Plants, Technology Innovation)
- SWOT Analysis of Key Players
Pricing Analysis for Ketone Testing Devices - Detailed Profiles of Major Companies
Abbott Laboratories
Roche Diagnostics
Medtronic
Bayer AG
Ascensia Diabetes Care
Lifescan
ACON Laboratories
Dexcom
Abbott Diabetes Care
Arkray
Trividia Health
Nova Biomedical
Xhale
Genteel
Ketonix
- Market Demand and Utilization
- Impact of Insurance Reimbursement Policies
- Consumer Adoption Trends in Home Care
- Regulatory and Compliance Factors
- Decision-Making Process for End-users
- By Value,2026-2030
- By Volume,2026-2030
- By Average Price,2026-2030


