Market Overview
The Philippines connected healthcare market—within which mobile monitoring devices (mHealth devices, RPM and clinical monitoring functions) are a core revenue engine—was valued at USD ~ million in the latest year, with historical build tracked over a multi-year series and forecasted expansion driven by telemedicine, mobile health apps, and wearable device uptake. In the prior year, market activity was already being reinforced by provider digitization initiatives and partnerships that pushed virtual care usage and device-led monitoring programs into mainstream workflows, creating a stronger base for device procurement and program bundling.
Metro Manila and other large urban clusters dominate demand because they concentrate tertiary hospital capacity, private provider networks, HMOs, and employer-funded care programs, which are the fastest adopters of connected workflows and monitoring protocols. These cities also have the densest after-sales service infrastructure (calibration, replacement, consumables) and the highest feasibility for integration with hospital HIS/EMR and clinician dashboards—key gating factors for clinical-grade mobile monitoring deployments. The expansion of digitally enabled care is further supported by national momentum around connected healthcare and the forecast growth outlook highlighted by Philippines connected healthcare commentary.
Market Segmentation
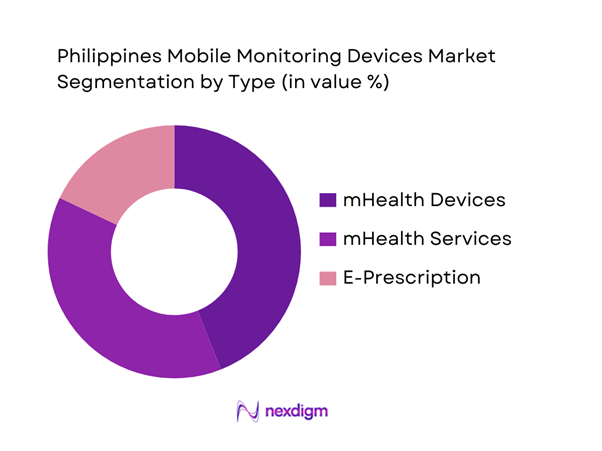
By Type
The market is segmented by type into mHealth services, mHealth devices, and e-prescription. mHealth devices typically dominate the “mobile monitoring devices” lens because procurement is triggered by measurable clinical workflows: remote vitals capture, chronic disease monitoring, post-discharge surveillance, and screening programs. Devices also create downstream recurring demand through accessories and consumables (cuffs, sensors, patches), replacement cycles, warranties, and service contracts—making them procurement-friendly for hospitals and scalable for payer programs. In the Philippines, device-led monitoring benefits from archipelagic care access constraints (reducing repeat facility visits) and the operational need to extend clinician oversight beyond facility walls. As payers and providers refine virtual-first pathways, device bundles paired with dashboards and triage services become easier to justify than standalone app-only offerings.
By Function Type
The market is also segmented by function into remote patient monitoring, clinical monitoring, telemedicine, and others. Remote patient monitoring tends to dominate the mobile monitoring devices opportunity because it directly converts device telemetry into actionable care workflows (alerts, escalation, nurse triage, care plan adjustments). It is also the function most frequently bundled into payer and employer chronic programs where outcomes (readmission reduction, adherence improvement, utilization control) can be tracked. Regional telehealth benchmarks also show RPM as a leading service component in the region’s telehealth revenue mix, reinforcing why RPM-linked device programs attract budget allocation faster than “nice-to-have” wellness monitoring. In the Philippines context, RPM is additionally supported by the need to serve patients outside urban cores and to keep facility load manageable while maintaining continuity of care.

Competitive Landscape
The Philippines mobile monitoring devices market is influenced by global medtech OEMs (clinical-grade monitoring, CGM, respiratory monitoring, hospital-to-home transition tools) and consumer wearable ecosystems increasingly used in hybrid monitoring programs. Competition often plays out through local distributors/integrators, after-sales service capability, clinician training, and integration readiness rather than hardware alone. The market’s competitive landscape analysis includes structure, positioning, and strategy benchmarking as part of the full report coverage.
| Company | Est. Year | HQ | Core Device Focus | PH Route-to-Market | Integration Readiness | Typical Buyer | After-Sales Model | Key Differentiator |
| Philips | 1891 | Netherlands | ~ | ~ | ~ | ~ | ~ | ~ |
| Medtronic | 1949 | Ireland | ~ | ~ | ~ | ~ | ~ | ~ |
| Abbott | 1888 | USA | ~ | ~ | ~ | ~ | ~ | ~ |
| Roche Diabetes Care | 1896 | Switzerland | ~ | ~ | ~ | ~ | ~ | ~ |
| Omron Healthcare | 1933 | Japan | ~ | ~ | ~ | ~ | ~ | ~ |
Philippines Mobile Monitoring Devices Market Dynamics and Performance Analysis
Growth Drivers
Chronic disease prevalence and monitoring demand
Chronic disease burden in the Philippines creates sustained “always-on” monitoring demand across hospitals, clinics, and home settings—especially for cardiometabolic and cardio-cerebrovascular risk that frequently needs longitudinal vitals trending (BP, SpO₂, ECG rhythm strips, glucose patterns, temperature) rather than episodic readings. Mortality data underscores the load: ischemic heart diseases accounted for ~ deaths in the country in a recent full-year reporting of causes of death, and PSA- referenced reporting for a more recent period cited ~ deaths attributed to ischemic heart diseases within the year’s first ten months—numbers that translate into high volumes of hypertensive, heart-failure, and arrhythmia follow-ups and post-event monitoring pathways where mobile devices are clinically useful (home BP cuffs, pulse oximeters, patch ECG, connected thermometry). This medical need is reinforced by macro capacity realities: the Philippines’ population reached ~ persons, expanding the absolute base of patients needing chronic monitoring and follow-ups, while national economic scale (GDP USD ~ billion) supports widening payer, employer, and provider spending on chronic care workflows that reduce avoidable admissions and deterioration.
Hospital bed optimization and early discharge protocols
Hospitals are structurally pressured to use beds for higher-acuity cases and discharge earlier when clinically safe, which elevates demand for mobile monitoring devices that “extend the ward” into step-down and home settings. Even without quoting bed-price economics, the constraint is visible in health system structure: DOH-supervised and controlled hospitals include ~ facilities, and licensed hospital rosters at regional level demonstrate a wide spread of small Level ~ type facilities where inpatient capacity is finite, making “monitor then discharge” pathways operationally attractive (post-surgery vitals checks, pneumonia recovery pulse-ox monitoring, HF weight and BP checks, maternal BP monitoring). In this context, devices become throughput tools: portable monitors enable earlier discharge with structured escalation (alerts, tele-nurse calls, return-to-ER triggers) and reduce length-of-stay pressure without diluting clinical governance. The macro overlay matters: with a population of ~, seasonal respiratory surges and chronic caseloads compete for limited staffed beds, while a GDP base of USD ~ billion reflects a health economy where private hospital networks increasingly differentiate via service lines (home recovery packages, RPM bundles) rather than only bricks-and-mortar expansion.
Challenges
Device regulatory approvals and compliance complexity
Regulatory compliance is a gating factor that shapes time-to-market, SKU strategy, and distributor economics for mobile monitoring devices. The Philippines’ environment is operationally complex because device categories can require different authorizations (registration and notification pathways, establishment licensing), and importation adds another compliance layer. For example, a Bureau of Customs memorandum circular clarifying authorizations for medical device importation explicitly requires a License to Operate as Medical Device Importer and Distributor during importation—raising the bar for channel partners and slowing down grey-market “easy imports” while increasing documentation burden for legitimate distributors. Market complexity is also reflected in the scale of the registrable ecosystem: a national medical device verification dataset referenced ~ medical devices recorded as of a recent cut, indicating a large, continuously updated universe of products that providers and procurement teams must validate (and that regulators must police). These compliance realities operate within the country’s macro context—population ~ and GDP USD ~ billion—because large demand attracts more entrants, increasing regulator workload and the volume of borderline and noncompliant products. For vendors, the challenge is not only approval; it is maintaining compliant labeling, UDI and traceability expectations where applicable, post-market vigilance, and distributor controls across a geographically dispersed market.
Data privacy and cybersecurity constraints
Mobile monitoring devices generate sensitive health data, and the Philippine compliance burden is heightened by active breach reporting and enforcement expectations. The National Privacy Commission reported ~ personal data breach notifications received, showing that breaches are not hypothetical; they are frequent enough to influence how hospitals, telemedicine providers, and employers evaluate device platforms. At the operational level, privacy constraints translate into mandatory governance artifacts: Data Protection Officer oversight, data mapping for device-to-app-to-cloud flows, consent and retention policies, vendor risk assessments, and incident response playbooks. Even where devices measure “simple vitals,” once data is tied to identifiable patients and used for clinical decisions or reimbursement documentation, it becomes high-risk personal data that must be secured. This pressure is magnified by scale: a population of ~ means more endpoints, more user accounts, more caregivers, and more opportunities for misconfiguration or credential compromise; and a GDP of USD ~ billion supports rapid digitization that can outpace governance maturity. The market impact is clear in purchasing behavior: health systems increasingly prefer vendors with strong encryption, role-based access controls, audit logs, PH-based or policy-compliant hosting options, and proven breach handling. Cybersecurity also becomes a field service issue—firmware patching, secure pairing, decommissioning devices, and preventing reuse without data wipes.
Opportunities
Hospital-at-home program expansion
Hospital-at-home expansion is a high-leverage growth opportunity because it structurally increases demand for multi-day, home-based monitoring kits and clinician dashboards—without requiring future statistics to justify it; the current system realities already support the case. The Philippines’ health system spans large public networks (including ~ DOH hospitals under supervision and control) plus extensive private hospital groups, and the archipelagic geography pushes providers to find scalable ways to extend care without forcing repeated facility visits. With a population of ~, the absolute number of patients who can benefit from monitored step-down pathways is large—post-infection recovery, chronic exacerbation watch, post-procedure observation, maternal BP monitoring—especially in urban catchments where travel time and outpatient congestion are high. Macro context reinforces feasibility: GDP at USD ~ billion supports the growth of private homecare services, logistics partners, and digital health operations that can run hospital-at-home models. The enabling ingredient is mobile monitoring device standardization: providers need kits that are easy to deploy, clinically validated, and serviceable, with escalation rules that protect clinical governance. The opportunity is most immediate where hospital-at-home reduces operational bottlenecks: freeing inpatient beds, shortening discharge cycles, and providing continuity monitoring for patients living far from tertiary centers.
Payer-led remote monitoring reimbursement models
Payer-led reimbursement is a growth opportunity because it converts remote monitoring from “optional add-on” to a reimbursable, standardized care component—driving repeatable demand across providers and accelerating device fleet scaling. The Philippines’ payer ecosystem is increasingly sensitive to measurable outcomes and documentation quality; mobile monitoring devices directly strengthen claims defensibility by producing timestamped readings tied to care plans and escalation actions. This opportunity sits on current macro scale: a ~ population creates high volume potential for payer programs, and GDP of USD ~ billion supports broader health financing capacity and the operationalization of digital claims and care coordination. Connectivity also makes the model practical: mobile reach at ~ subscriptions per ~ people supports patient messaging, adherence nudges, and data transmission for RPM programs in many settings. At the same time, privacy risk is a gating design constraint—NPC recorded ~ personal data breach notifications—so payers and their provider networks will favor RPM models that embed privacy-by-design (consent, minimization, encryption, audit trails) and vendor accountability. The near-term commercial implication is that device vendors who can align product evidence outputs to payer documentation needs—standard reports, exception logs, adherence summaries, clinician notes support—are more likely to be included in reimbursed care pathways.
Future Outlook
Over the next five years, the Philippines mobile monitoring devices ecosystem is expected to expand as providers standardize hospital-to-home protocols, payers build structured chronic care bundles, and enterprise buyers push workforce screening and prevention programs. The most scalable models will be bundled deployments (device + app + clinician dashboard + triage) with clear KPIs—alert quality, adherence, escalation rates, and measurable reductions in avoidable utilization. The forecast outlook for connected healthcare—covering device and monitoring functions—supports a high-growth trajectory as digital health adoption and government support continue to strengthen.
Major Players
- Medtronic
- Philips
- GE HealthCare
- Abbott
- Roche Diabetes Care
- Dexcom
- Masimo
- Omron Healthcare
- ResMed
- iHealth
- Withings
- Garmin
- Samsung
- Apple
Key Target Audience
- Hospitals and Integrated Health Systems
- Health Maintenance Organizations / Private Insurers
- PhilHealth and public payer stakeholders
- Government and regulatory bodies
- Corporate employers and occupational health program owners
- Home healthcare providers and post-acute care networks
- Pharmacy chains and retail health operators
- Investments and venture capitalist firms
Research Methodology
Step 1: Identification of Key Variables
We build a Philippines-specific ecosystem map covering OEMs, local distributors, hospital groups, HMOs, pharmacies, and telehealth platforms. Desk research consolidates regulatory pathways (PH FDA device registration), channel structures, and typical buyer decision criteria to define variables such as installed base, procurement cycles, and integration requirements.
Step 2: Market Analysis and Construction
We compile historical revenues and unit shipments using a hybrid approach: distributor sell-in checks, hospital procurement patterns, channel price bands (ASP), and program-based monitoring contracts. We normalize device ASPs across clinical-grade versus consumer-grade and reconcile volumes against active connected device estimates.
Step 3: Hypothesis Validation and Expert Consultation
We validate assumptions through structured interviews with hospital biomedical leads, payer chronic program owners, distributor product heads, and clinician champions. CATI-style consultations focus on workflow adoption, alert management, integration barriers, and service-level performance that directly affects renewals.
Step 4: Research Synthesis and Final Output
We triangulate top-down connected healthcare benchmarks with bottom-up device and program builds, then finalize segment splits, competitive benchmarking, and forecast narratives. Final outputs emphasize deployability—pilots, conversion KPIs, and partner models suited for archipelagic service delivery.
- Executive Summary
- Research Methodology (Market definitions and inclusions/exclusions, assumptions, abbreviations, primary interview universe, stakeholder mapping, top-down triangulation, bottom-up revenue build, device ASP normalization, channel checks, tender and purchase order tracking, regulatory validation approach, limitations)
- Definition and Scope
- Market Genesis and Evolution Timeline
- Healthcare Delivery and Referral Architecture
- Care Settings Map
- Value Chain and Supply Chain
- Growth Drivers
Chronic disease prevalence and monitoring demand
Hospital bed optimization and early discharge protocols
Expansion of telemedicine and virtual care workflows
Employer-led preventive health programs
Mobile and broadband connectivity penetration - Challenges
Device regulatory approvals and compliance complexity
Data privacy and cybersecurity constraints
Integration challenges with hospital information systems
After-sales service and device maintenance reach
Counterfeit and grey-market device circulation - Opportunities
Hospital-at-home program expansion
Payer-led remote monitoring reimbursement models
Device and platform bundled offerings
AI-enabled alert management and triage
Rural and island-region healthcare access - Trends
Subscription-based monitoring programs
Device leasing and managed service models
Pharmacy-led health screening integration
Consumer wearables in hybrid clinical workflows - Regulatory & Policy Landscape
- SWOT Analysis
- Stakeholder & Ecosystem Analysis
- Porter’s Five Forces Analysis
- Competitive Intensity & Ecosystem Mapping
- By Value, 2019–2024
- By Volume, 2019–2024
- By Average Selling Price, 2019–2024
- Installed Base and Active Connected Devices, 2019–2024
- By Fleet Type (in Value %)
Remote patient monitoring kits
Wearable monitoring devices
Portable vital sign monitors
Specialty monitoring devices
Multiparameter monitoring devices - By Application (in Value %)
Chronic disease management
Post-acute discharge monitoring
Maternal and newborn monitoring
Infectious disease follow-up
Preventive wellness programs - By Technology Architecture (in Value %)
Single-parameter devices
Multi-parameter integrated devices
Patch-based continuous monitoring
Gateway-based monitoring systems - By Connectivity Type (in Value %)
Bluetooth-enabled
Wi-Fi enabled
Cellular and eSIM enabled
IoT gateway based
Offline sync and store-and-forward - By End-Use Industry (in Value %)
Hospitals
Clinics and ambulatory care centers
Home healthcare providers
Corporate and employer clinics
Retail and direct-to-consumer - By Region (in Value %)
Metro Manila and NCR
CALABARZON
Central Luzon
Central Visayas
Davao Region
- Market Structure
- Cross Comparison Parameters (installed base in the Philippines, clinical-grade validation breadth, FDA registration coverage, interoperability depth, battery life and uptime service-level agreements, after-sales footprint and turnaround time, program pricing model, alert accuracy and false-alarm suppression capability)
- Competitive Positioning Matrix
- SWOT Analysis of Key Players
- Key Winning Strategies
- Detailed Profiles of Major Companies
Medtronic
Philips
GE HealthCare
Abbott
Roche Diabetes Care
Dexcom
Masimo
Omron Healthcare
ResMed
iHealth
Withings
Garmin
Samsung
Apple
Huawei
- Hospital procurement and validation workflows
- HMO and payer adoption logic
- Corporate and employer program demand
- Home healthcare provider requirements
- Purchasing constraints and pain points
- By Value, 2025–2030
- By Volume, 2025–2030
- By Average Selling Price, 2025–2030
- Installed Base and Active Connected Devices, 2025–2030


