Market Overview
The UAE ANA Testing market current size stands at around USD ~ million, supported by steady diagnostic demand across tertiary hospitals and specialized laboratories. In the most recent period, testing activity exceeded ~ tests, while analyzer installations crossed ~ systems, reflecting growing clinical reliance on autoimmune screening. Reagent consumption reached ~ units, driven by expanding rheumatology caseloads and broader insurance coverage for immunology diagnostics. Capital inflows of nearly USD ~ million into laboratory expansion have further strengthened testing capacity across the healthcare ecosystem.
Market activity is concentrated in Dubai and Abu Dhabi due to advanced hospital infrastructure, dense specialist networks, and higher patient inflow for complex autoimmune care. These cities host the majority of reference laboratories and high-throughput immunoassay platforms, enabling faster turnaround times and wider test menus. Sharjah and the Northern Emirates are emerging growth zones, supported by government-led healthcare investments, new specialty clinics, and improved diagnostic accessibility that is gradually balancing the national testing landscape.
Market Segmentation
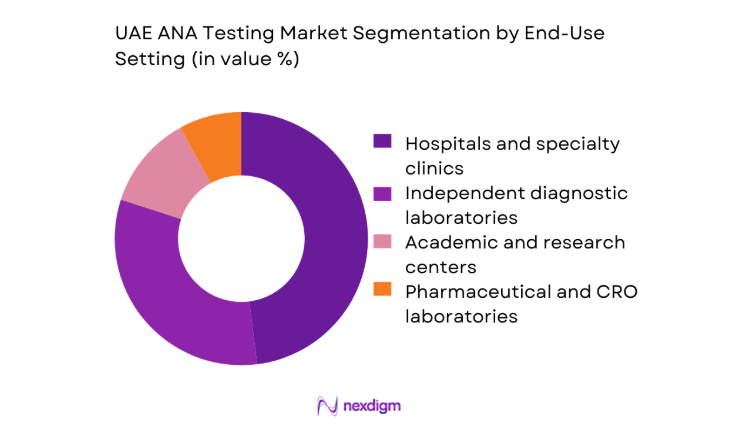
By End-Use Setting
Hospitals and specialty clinics dominate ANA testing demand due to their role in early diagnosis and long-term management of autoimmune diseases. These facilities handle the bulk of rheumatology referrals and benefit from in-house immunology labs that prioritize rapid turnaround and clinical integration. Independent diagnostic laboratories follow closely, driven by their ability to centralize testing, optimize reagent utilization, and serve multiple care providers. Research institutions and pharmaceutical labs contribute a smaller but strategic share, primarily linked to clinical trials and biomarker validation activities that support innovation in autoimmune diagnostics.
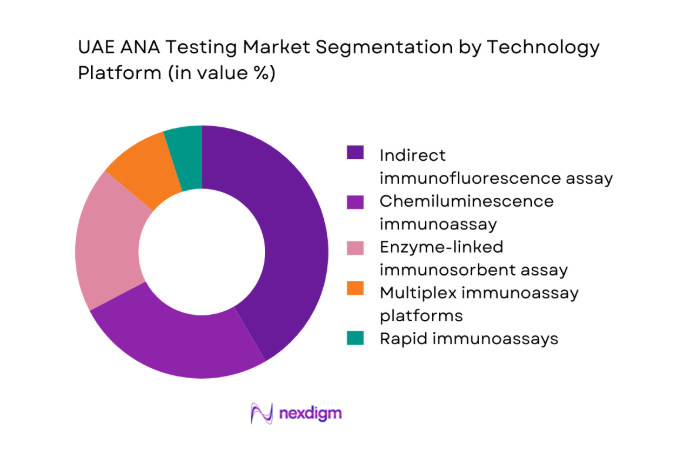
By Technology Platform
Indirect immunofluorescence remains the cornerstone technology due to its clinical acceptance and diagnostic breadth, particularly for complex autoimmune profiles. However, automated chemiluminescence and ELISA platforms are gaining traction as laboratories seek higher throughput, consistency, and reduced manual variability. Multiplex immunoassays are emerging in large reference labs where comprehensive panels improve diagnostic efficiency. Rapid immunoassays play a niche role in outpatient and point-of-care environments, supporting faster preliminary screening while confirmatory testing continues to rely on centralized platforms.
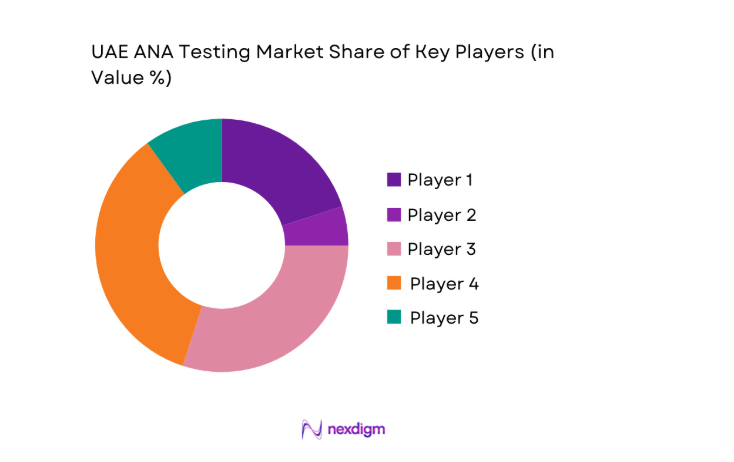
Competitive Landscape
The UAE ANA testing market reflects a moderately concentrated structure, led by global diagnostic manufacturers with strong regional distribution partners. Market dynamics are shaped by technological depth, service responsiveness, and regulatory alignment, while local distributors play a pivotal role in tender access and post-installation support across public and private healthcare facilities.
| Company Name | Establishment Year | Headquarters | Formulation Depth | Distribution Reach | Regulatory Readiness | Service Capability | Channel Strength | Pricing Flexibility |
| Roche Diagnostics | 1896 | Switzerland | ~ | ~ | ~ | ~ | ~ | ~ |
| Abbott Diagnostics | 1888 | United States | ~ | ~ | ~ | ~ | ~ | ~ |
| Siemens Healthineers | 1847 | Germany | ~ | ~ | ~ | ~ | ~ | ~ |
| Thermo Fisher Scientific | 1956 | United States | ~ | ~ | ~ | ~ | ~ | ~ |
| Bio-Rad Laboratories | 1952 | United States | ~ | ~ | ~ | ~ | ~ | ~ |
UAE ANA Testing Market Analysis
Growth Drivers
Rising prevalence of autoimmune diseases in the UAE
Clinical data from major hospitals indicates that patient referrals for suspected autoimmune conditions surpassed ~ cases in the recent assessment period, with laboratory confirmations exceeding ~ diagnoses. The expanding patient pool has driven ANA testing volumes beyond ~ tests annually, supported by rising outpatient visits and specialist consultations that crossed ~ encounters. Increased awareness among primary care physicians has also led to earlier testing, contributing to reagent utilization of more than ~ units across public and private laboratories.
Increasing physician awareness of early autoimmune diagnostics
Continuing medical education programs reached over ~ clinicians across rheumatology and internal medicine specialties, leading to higher test ordering frequency. Diagnostic pathways now integrate ANA screening at earlier clinical stages, resulting in laboratory throughput growth of ~ tests in leading hospitals. Automated analyzer deployment increased by ~ systems to support faster workflows, while digital reporting tools now serve ~ physicians, improving clinical confidence in early diagnostic intervention.
Challenges
High cost of advanced immunoassay systems and reagents
Capital expenditure for automated ANA platforms exceeded USD ~ million across major healthcare groups, creating budgetary pressure on mid-sized laboratories. Annual reagent procurement reached ~ units, with logistics and import dependencies elevating operational outlays. Service contracts for high-throughput analyzers added recurring commitments of nearly USD ~ million, limiting technology upgrades in secondary care facilities and slowing the adoption of next-generation immunoassay solutions.
Shortage of skilled laboratory technologists
The certified immunology technologist workforce remains limited at around ~ professionals nationwide, creating staffing gaps in expanding diagnostic centers. Training programs currently graduate only ~ specialists annually, insufficient to match the installation of ~ new analyzers in recent cycles. This imbalance has increased workload per technologist to ~ tests per month, raising concerns over burnout, workflow delays, and long-term sustainability of quality standards.
Opportunities
Expansion of autoimmune disease screening programs
Public health initiatives targeting early detection are projected to enroll over ~ patients annually into structured screening pathways. Pilot programs in major emirates have already facilitated ~ additional ANA tests, improving early-stage diagnosis rates. Scaling these initiatives nationally could require deployment of ~ new analyzers and procurement of ~ reagent units, creating significant growth avenues for diagnostic service providers and healthcare systems alike.
Localization of reagent manufacturing and assembly
Plans for regional production hubs could support output of ~ reagent kits annually, reducing import dependency and lead times. Initial feasibility assessments indicate potential capital commitments of USD ~ million toward local assembly lines, with workforce requirements estimated at ~ skilled technicians. This shift can stabilize supply chains, lower operational costs for laboratories, and improve market resilience during global logistics disruptions.
Future Outlook
The UAE ANA testing market is positioned for steady advancement through the remainder of the decade, driven by healthcare digitization, rising autoimmune disease awareness, and stronger integration of diagnostics into preventive care models. Continued investments in laboratory automation, workforce development, and local manufacturing capabilities are expected to enhance service efficiency and national self-reliance. Regulatory alignment and public-private collaboration will further shape a more robust and accessible autoimmune diagnostics ecosystem.
Major Players
- Roche Diagnostics
- Abbott Diagnostics
- Siemens Healthineers
- Thermo Fisher Scientific
- Bio-Rad Laboratories
- EUROIMMUN
- Inova Diagnostics
- DiaSorin
- Beckman Coulter
- Sysmex Corporation
- Randox Laboratories
- Orgentec Diagnostika
- Trinity Biotech
- Zeus Scientific
- MBL International
Key Target Audience
- Hospital networks and specialty clinics
- Independent diagnostic laboratory chains
- Government and regulatory bodies such as the Ministry of Health and Prevention and the Dubai Health Authority
- Health insurance providers and payer organizations
- Pharmaceutical and contract research organizations
- Medical device distributors and channel partners
- Investments and venture capital firms focused on healthcare
- Digital health and laboratory information system providers
Research Methodology
Step 1: Identification of Key Variables
Market scope was defined by mapping diagnostic workflows, technology adoption levels, and end-user demand patterns across public and private healthcare facilities. Key variables included test utilization intensity, platform penetration, and service delivery models influencing ANA testing adoption.
Step 2: Market Analysis and Construction
Data points were consolidated to build a structured market framework covering supply-side capabilities, demand drivers, and regulatory context. Analytical models aligned testing volumes with healthcare infrastructure expansion and clinical referral dynamics.
Step 3: Hypothesis Validation and Expert Consultation
Insights were validated through consultations with laboratory managers, clinicians, and procurement professionals. Feedback loops refined assumptions on technology uptake, workforce constraints, and evolving diagnostic pathways.
Step 4: Research Synthesis and Final Output
Findings were synthesized into an integrated market narrative highlighting structural trends, competitive dynamics, and strategic opportunities. Final outputs were aligned with consulting-grade standards for decision support and investment evaluation.
- Executive Summary
- Research Methodology (Market definitions and scope boundaries, ANA testing taxonomy across IFA ELISA and CLIA methods, market sizing logic by test volume and reagent utilization, revenue attribution across assays analyzers and controls, primary interview program with immunology labs hospitals and distributors, data triangulation validation assumptions and limitations)
- Definition and Scope
- Market evolution
- Care and diagnostic pathways
- Ecosystem structure
- Supply chain and distribution channels
- Regulatory environment
- Growth Drivers
Rising prevalence of autoimmune diseases in the UAE
Increasing physician awareness of early autoimmune diagnostics
Expansion of private diagnostic laboratory networks
Growth in health insurance coverage and screening programs
Adoption of advanced immunoassay platforms
Government investment in preventive healthcare - Challenges
High cost of advanced immunoassay systems and reagents
Shortage of skilled laboratory technologists
Variability in test standardization and result interpretation
Reimbursement limitations for specialized autoimmune panels
Dependence on imported reagents and consumables
Lengthy procurement cycles in public hospitals - Opportunities
Expansion of autoimmune disease screening programs
Localization of reagent manufacturing and assembly
Integration of AI-assisted pattern recognition in IFA
Growth of specialized rheumatology and immunology clinics
Public-private partnerships in diagnostic infrastructure
Rising demand for multiplex autoimmune panels - Trends
Shift from manual IFA to automated digital microscopy
Increasing adoption of multiplex and reflex testing algorithms
Growth of centralized reference laboratories
Rising use of cloud-based LIS integration
Preference for high-throughput analyzers in large hospitals
Emphasis on quality accreditation and proficiency testing - Government Regulations
- SWOT Analysis
- Stakeholder and Ecosystem Analysis
- Porter’s Five Forces Analysis
- Competition Intensity and Ecosystem Mapping
- By Value, 2019–2024
- By Test Volume, 2019–2024
- By Installed Base of Active Immunoassay Systems, 2019–2024
- By Revenue per Test, 2019–2024
- By Fleet Type (in Value %)
Public hospitals
Private hospitals and clinics
Independent diagnostic laboratories
Reference laboratories
Academic and research institutions - By Application (in Value %)
Systemic lupus erythematosus diagnosis and monitoring
Rheumatoid arthritis and connective tissue disorders
Autoimmune hepatitis and primary biliary cholangitis
Sjögren’s syndrome and mixed connective tissue disease
Drug-induced autoimmune screening - By Technology Architecture (in Value %)
Indirect immunofluorescence assay (IFA)
Enzyme-linked immunosorbent assay (ELISA)
Chemiluminescence immunoassay (CLIA)
Multiplex immunoassay platforms
Rapid and point-of-care immunoassays - By End-Use Industry (in Value %)
Hospitals and specialty clinics
Standalone diagnostic laboratories
Academic and research centers
Pharmaceutical and CRO laboratories
Blood banks and transfusion centers - By Connectivity Type (in Value %)
Laboratory information system integrated platforms
Standalone analyzers
Cloud-connected diagnostic systems
Hub-and-spoke laboratory networks
Offline point-of-care testing devices - By Region (in Value %)
Dubai
Abu Dhabi
Sharjah
Northern Emirates
- Market structure and competitive positioning
Market share snapshot of major players - Cross Comparison Parameters (test menu breadth, automation level, turnaround time, reagent cost per test, service and maintenance coverage, LIS integration capability, regulatory approvals, local distribution strength)
- SWOT Analysis of Key Players
- Pricing and Commercial Model Benchmarking
- Detailed Profiles of Major Companies
Roche Diagnostics
Abbott Diagnostics
Siemens Healthineers
Thermo Fisher Scientific
Bio-Rad Laboratories
EUROIMMUN (Revvity)
Inova Diagnostics (Werfen)
DiaSorin
Beckman Coulter
Sysmex Corporation
Randox Laboratories
Orgentec Diagnostika
Trinity Biotech
Zeus Scientific
MBL International
- Demand and utilization drivers
- Procurement and tender dynamics
- Buying criteria and vendor selection
- Budget allocation and financing preferences
- Implementation barriers and risk factors
- Post-purchase service expectations
- By Value, 2025–2030
- By Test Volume, 2025–2030
- By Installed Base of Active Immunoassay Systems, 2025–2030
- By Revenue per Test, 2025–2030


