Market Overview
The UAE Respiratory Rate Monitors Equipment market current size stands at around USD ~ million, reflecting sustained demand across acute care and perioperative settings driven by modernization of patient monitoring infrastructure and rising acuity in hospital case mixes. Procurement is shaped by replacement of legacy bedside monitors, expansion of step-down units, and integration with centralized monitoring platforms. Service contracts, interoperability requirements, and clinical training programs influence vendor selection, while lifecycle management practices anchor long-term utilization within hospital networks.
Demand concentration is strongest in Abu Dhabi and Dubai due to dense tertiary care clusters, advanced ICU capacity, and higher penetration of digital hospital programs. Northern Emirates show growing uptake aligned with public hospital upgrades and private network expansions. Ecosystem maturity varies by emirate, reflecting distributor footprint, service coverage, and biomedical engineering capacity. Policy frameworks emphasizing patient safety, device interoperability, and continuity of care support adoption across acute, ambulatory, and homecare pathways.
Market Segmentation
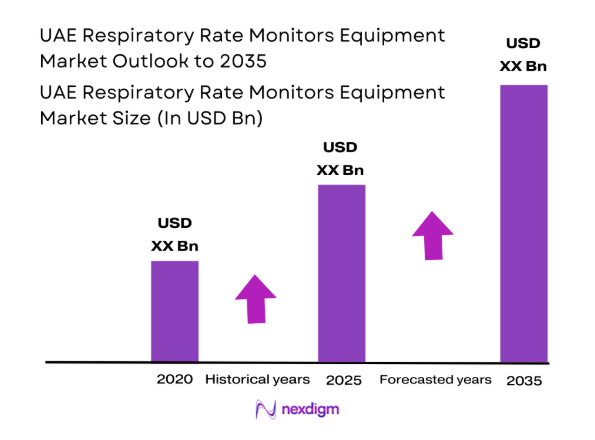
By Product Type
Standalone and multiparameter systems dominate procurement decisions as hospitals prioritize continuous monitoring across ICU, operating rooms, and step-down wards. Multiparameter platforms are favored due to consolidation of vital sign streams into centralized stations, reducing alarm fatigue and enabling early warning protocols. Wearable and contactless devices show faster uptake in post-acute and homecare settings where mobility and infection control are critical. Capnography-based solutions remain concentrated in anesthesia and emergency workflows. Technology selection reflects clinical acuity, interoperability with existing monitors, and lifecycle serviceability within hospital biomedical teams.
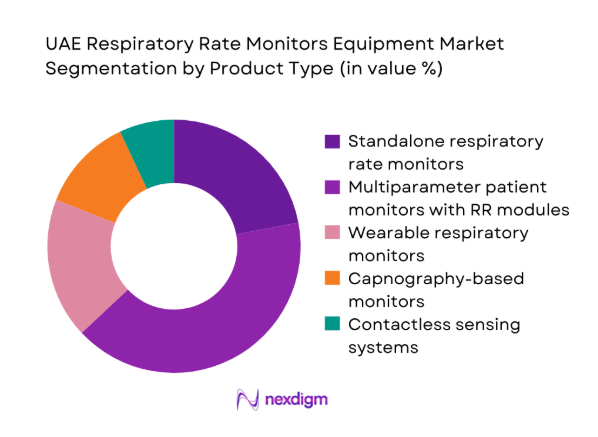
By Care Setting
Acute care settings lead demand due to higher monitoring intensity, staffing models, and compliance with patient safety protocols. ICUs and operating rooms prioritize continuous respiratory surveillance integrated with alarms and documentation systems. General wards adopt step-down monitoring to detect deterioration earlier, supported by centralized telemetry. Emergency departments require rapid triage and short-cycle monitoring. Homecare adoption is rising as chronic respiratory management and post-discharge monitoring expand, supported by remote connectivity and service models. Allocation across settings reflects acuity mix, bed capacity growth, and digital workflow maturity.
Competitive Landscape
The competitive environment features global technology providers supported by local distribution partners and service integrators. Differentiation centers on clinical reliability, integration with hospital IT, service responsiveness, and regulatory readiness. Tender-driven procurement emphasizes lifecycle support and training coverage alongside device performance.
| Company Name | Establishment Year | Headquarters | Formulation Depth | Distribution Reach | Regulatory Readiness | Service Capability | Channel Strength | Pricing Flexibility |
| Philips Healthcare | 1891 | Netherlands | ~ | ~ | ~ | ~ | ~ | ~ |
| GE HealthCare | 1994 | United States | ~ | ~ | ~ | ~ | ~ | ~ |
| Medtronic | 1949 | Ireland | ~ | ~ | ~ | ~ | ~ | ~ |
| Masimo | 1989 | United States | ~ | ~ | ~ | ~ | ~ | ~ |
| Dräger | 1889 | Germany | ~ | ~ | ~ | ~ | ~ | ~ |
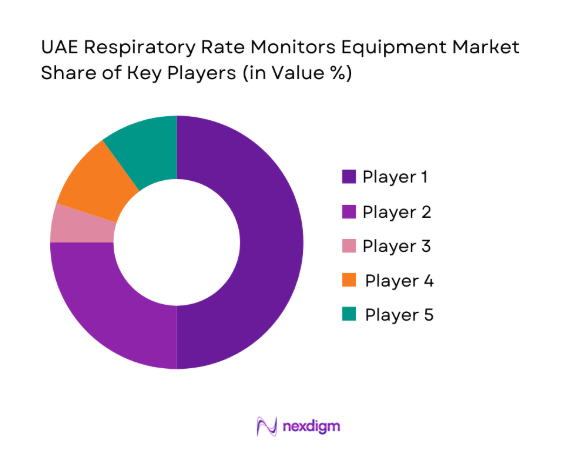
UAE Respiratory Rate Monitors Equipment Market Analysis
Growth Drivers
Rising ICU bed capacity and critical care infrastructure expansion
ICU capacity expanded across tertiary hospitals, with new beds commissioned in Abu Dhabi and Dubai during 2023 and 2024 to support higher acuity case mixes. Emergency admissions rose by 214000 in 2024 across public networks, increasing demand for continuous respiratory surveillance in triage and stabilization bays. Operating room throughput increased by 68000 procedures in 2023, driving perioperative monitoring utilization. Hospital digital transformation programs added 146 centralized monitoring stations in 2024, strengthening real-time respiratory surveillance. National patient safety initiatives emphasized early deterioration detection protocols, reinforcing continuous respiratory rate monitoring across critical pathways.
Increasing burden of respiratory disorders and post-COVID monitoring protocols
Respiratory-related admissions in tertiary facilities increased by 39200 in 2023 and 41800 in 2024, driven by chronic obstructive conditions, asthma exacerbations, and post-infectious complications. Post-discharge follow-up clinics recorded 126000 respiratory assessments in 2024, elevating monitoring intensity beyond acute settings. Emergency department presentations for dyspnea rose by 17400 in 2023, reinforcing triage monitoring needs. Clinical pathways standardized continuous respiratory surveillance for high-risk cohorts across 58 hospital wards in 2024. Institutional protocols expanded monitoring coverage to step-down units, increasing utilization of respiratory rate monitoring capabilities across care transitions.
Challenges
High capital costs of advanced multiparameter monitors
Capital planning cycles in public hospitals lengthened during 2023 and 2024 as equipment refresh programs competed with imaging and surgical upgrades. Biomedical departments reported 312 deferred replacements of bedside monitors in 2024 due to competing capital priorities. Operating theaters added 27 new rooms in 2023, stretching capital allocation across anesthesia workstations and monitoring upgrades. Procurement committees increased scrutiny on total lifecycle ownership metrics across 64 tenders in 2024, slowing award timelines. Facility expansions in secondary hospitals prioritized bed capacity over device refresh, delaying modernization of respiratory monitoring platforms despite clinical need pressures.
Lengthy procurement cycles in public sector tenders
Public tender cycles averaged 214 days in 2024 across major hospital networks, delaying deployment of updated monitoring technologies. Compliance documentation requirements expanded to 19 mandatory files per bid in 2023, increasing administrative burden for procurement teams. Bid evaluation committees reviewed 142 technical submissions in 2024 for monitoring upgrades, extending approval timelines. Contract award appeals occurred in 11 instances during 2023, pausing project execution. Installation schedules slipped by 68 days on average due to site readiness constraints, delaying clinical access to updated respiratory monitoring capabilities in high-acuity wards.
Opportunities
Expansion of step-down unit monitoring protocols in private hospitals
Private hospital networks expanded step-down capacity by 186 beds in 2023 and 214 beds in 2024 to manage post-ICU transitions. Clinical governance committees standardized early warning protocols across 41 wards in 2024, increasing continuous respiratory surveillance adoption. Nurse-to-patient ratios improved by 1.2 points in monitored wards, enabling sustained device utilization. Central monitoring hubs added 23 new stations in 2023, extending respiratory telemetry coverage. Accreditation cycles in 2024 reinforced continuous monitoring criteria for high-risk patients, supporting broader deployment of respiratory rate monitoring across post-acute care pathways.
Growing demand for wearable and contactless respiratory monitoring solutions
Homecare enrollments for chronic respiratory management increased by 16800 patients in 2023 and 19300 in 2024 across major provider networks. Post-discharge programs integrated remote monitoring protocols across 26 clinics in 2024, extending respiratory surveillance beyond inpatient settings. Infection control committees expanded contactless monitoring pilots across 9 neonatal units in 2023. Remote care coordinators handled 74000 follow-up interactions in 2024, reinforcing need for continuous respiratory data streams. Digital health roadmaps approved in 2023 prioritized wearable integration into care pathways, enabling scalable monitoring adoption across ambulatory and homecare environments.
Future Outlook
The market trajectory reflects sustained modernization of acute care monitoring, deeper integration with digital hospital platforms, and expanding post-acute surveillance. Adoption will broaden across step-down and homecare pathways as protocols mature and interoperability improves. Regulatory emphasis on patient safety and continuity of care will continue to shape procurement priorities and service models.
Major Players
- Philips Healthcare
- GE HealthCare
- Medtronic
- Masimo
- Dräger
- Nihon Kohden
- Mindray
- Spacelabs Healthcare
- Schiller
- Nonin Medical
- Fukuda Denshi
- Welch Allyn
- Edan Instruments
- Contec Medical Systems
- Becton Dickinson
Key Target Audience
- Public hospital procurement authorities in Abu Dhabi and Dubai health systems
- Private hospital networks and specialty care providers
- Home healthcare service operators and remote monitoring programs
- Medical device distributors and authorized channel partners
- Biomedical engineering and clinical technology management teams
- Investments and venture capital firms
- Government and regulatory bodies including MOHAP, DHA, and DoH Abu Dhabi
- Digital health platform integrators and health IT providers
Research Methodology
Step 1: Identification of Key Variables
Clinical use-cases, care settings, technology modalities, interoperability needs, and service requirements were defined to structure the assessment framework. Regulatory pathways, procurement mechanisms, and channel structures were mapped to contextualize adoption dynamics. Installed base maturity and replacement cycles informed utilization patterns.
Step 2: Market Analysis and Construction
Demand drivers across ICU, perioperative, ward, and homecare pathways were analyzed to construct utilization scenarios. Ecosystem roles across OEMs, distributors, and service partners were mapped to identify access points. Policy and accreditation requirements were aligned with clinical workflow integration.
Step 3: Hypothesis Validation and Expert Consultation
Clinical operations leaders and biomedical managers validated workflow integration assumptions and utilization constraints. Procurement specialists reviewed tender dynamics and evaluation criteria shaping vendor selection. Service managers assessed deployment readiness and post-installation performance requirements.
Step 4: Research Synthesis and Final Output
Findings were synthesized into segment-level narratives aligned with care pathways and technology modalities. Constraints and enablers were stress-tested against institutional indicators and policy direction. Implications were consolidated into actionable insights for stakeholders across the ecosystem.
- Executive Summary
- Research Methodology (Market Definitions and clinical use-cases for respiratory rate monitoring devices, Primary interviews with ICU clinicians biomedical engineers and hospital procurement heads across UAE, Analysis of UAE hospital procurement tenders and GPO purchase data for patient monitoring equipment, Distributor and OEM shipment tracking for bedside and wearable respiratory monitors in UAE, Regulatory and standards review with MOHAP DHA and DoH Abu Dhabi device registries
- Definition and Scope
- Market evolution
- Clinical usage pathways across acute care step-down and homecare
- Ecosystem structure of OEMs distributors integrators and care providers
- Supply chain and channel structure
- Regulatory environment and device approval pathways in UAE
- Growth Drivers
Rising ICU bed capacity and critical care infrastructure expansion
Increasing burden of respiratory disorders and post-COVID monitoring protocols
Adoption of continuous vital signs monitoring in general wards
Government investments in digital health and smart hospital initiatives
Growth of homecare and remote patient monitoring programs
Technology upgrades toward multiparameter and wireless monitoring systems - Challenges
High capital costs of advanced multiparameter monitors
Lengthy procurement cycles in public sector tenders
Interoperability issues with existing hospital IT and EMR systems
Shortage of trained biomedical and clinical staff for device utilization
Regulatory compliance and registration timelines with UAE authorities
Price sensitivity and budget constraints in mid-tier private hospitals - Opportunities
Expansion of step-down unit monitoring protocols in private hospitals
Growing demand for wearable and contactless respiratory monitoring solutions
Localization of distribution and after-sales service capabilities
Integration with remote patient monitoring platforms for homecare
Replacement demand for aging installed base in legacy hospitals
Public-private partnerships for ICU capacity expansion - Trends
Shift toward wireless and wearable respiratory monitoring
Integration of respiratory rate data into centralized patient monitoring platforms
Adoption of contactless sensing in neonatal and infectious disease wards
Bundling of respiratory rate monitoring with multiparameter systems
Increased emphasis on early warning score systems and continuous monitoring
Service contracts and managed equipment services gaining traction - Government Regulations
- SWOT Analysis
- Stakeholder and Ecosystem Analysis
- Porter’s Five Forces Analysis
- Competition Intensity and Ecosystem Mapping
- By Value, 2019–2024
- By Volume, 2019–2024
- By Installed Base, 2019–2024
- By Average Selling Price, 2019–2024
- By Product Type (in Value %)
Standalone respiratory rate monitors
Multiparameter patient monitors with respiratory rate modules
Wearable respiratory rate monitoring devices
Capnography-based respiratory rate monitors
Acoustic and impedance-based respiratory monitors - By Technology (in Value %)
Impedance pneumography
Capnography
Acoustic respiration monitoring
Radar and contactless sensing
Photoplethysmography-derived respiration - By Care Setting (in Value %)
Intensive care units
Operating rooms and PACU
General wards and step-down units
Emergency departments
Homecare and remote patient monitoring - By End User (in Value %)
Public hospitals
Private hospitals
Ambulatory surgical centers
Home healthcare providers
Defense and government medical facilities - By Distribution Channel (in Value %)
Direct OEM sales
Authorized medical device distributors
Group purchasing organizations
Tender-based government procurement
E-commerce and digital procurement platforms
- Market structure and competitive positioning
- Market share snapshot of major players
- Cross Comparison Parameters (Product portfolio breadth, Local service footprint, Pricing competitiveness, Regulatory approvals in UAE, Integration with hospital IT systems, Warranty and AMC coverage, Distributor network strength, Clinical training and support)
- SWOT Analysis of Key Players
- Pricing and Commercial Model Benchmarketing
- Detailed Profiles of Major Companies
Philips Healthcare
GE HealthCare
Medtronic
Masimo
Dräger
Nihon Kohden
Mindray
Fukuda Denshi
Spacelabs Healthcare
Nonin Medical
Schiller
Becton Dickinson
Contec Medical Systems
Edan Instruments
Welch Allyn
- Demand and utilization drivers
- Procurement and tender dynamics
- Buying criteria and vendor selection
- Budget allocation and financing preferences
- Implementation barriers and risk factors
- Post-purchase service expectations
- By Value, 2025–2030
- By Volume, 2025–2030
- By Installed Base, 2025–2030
- By Average Selling Price, 2025–2030