Market Overview
The USA Machine Learning in Healthcare market sits inside the broader U.S. AI-in-healthcare spend, valued at USD ~ billion, and is propelled by data-rich care delivery, enterprise digitization, and automation demand across clinical and administrative workflows. The scale of U.S. healthcare expenditure provides the commercial “surface area” for ML deployment: national health spending reached USD ~ trillion, while per-capita spending was about USD ~, with an expected rise to USD ~, expanding incentives to improve productivity, reduce avoidable utilization, and strengthen coding/claims integrity.
The United States dominates because it concentrates (i) hyperscalers and AI platforms, (ii) large integrated delivery networks and payers with large datasets, and (iii) an unusually deep venture pipeline for clinical and workflow AI. Commercial gravity clusters around San Jose, Seattle, and San Francisco (high AI job creation intensity), alongside life-sciences-heavy hubs such as Boston–Cambridge, where dense research-to-clinic pathways accelerate validation and adoption. Capital formation reinforces this: U.S. startups raised USD ~ billion, and healthtech funding—after dipping to USD ~ billion—began rebounding as AI-led ROI narratives improved.
Market Segmentation
By Technology
The USA Machine Learning in Healthcare market is segmented into Machine Learning and Non-ML AI (NLP, Computer Vision, and Context-Aware Computing). Machine Learning leads because it is the most broadly commercialized “workhorse” across payer, provider, pharma, and device workflows—risk prediction, patient deterioration flags, readmission propensity, utilization management, operational forecasting, and clinical pathway optimization. ML also aligns well with the realities of U.S. data estates: structured EHR fields, claims histories, and time-series vitals are common inputs for supervised and deep learning pipelines, enabling faster iteration cycles than net-new data modalities. In addition, ML’s measurable KPI fit (alert performance, throughput, denial reduction, length-of-stay optimization) maps cleanly to U.S. procurement logic, which increasingly requires provable ROI, governance artifacts, and monitoring plans before enterprise rollouts.
By End User
The USA Machine Learning in Healthcare market is segmented into Pharmaceutical & Biotechnology Companies and Other End Users (providers, payers, patients, and others). Pharma & biotech leads because ML delivers direct economic leverage in high-cost, high-cycle processes: target discovery, lead optimization, biomarker identification, trial design, patient stratification, and protocol feasibility—areas where time saved and probability-of-success improvements translate into outsized value. These organizations often operate with centralized data science groups, clearer analytics budgets, and mature governance models, which reduces friction compared with multi-stakeholder provider deployments that require deep workflow redesign and clinician adoption. In parallel, ML-enabled clinical trials analytics and real-world evidence pipelines increasingly depend on scalable models that can be reused across programs, making the revenue pool for repeatable ML platforms and partnerships structurally attractive in the U.S. ecosystem.
Competitive Landscape
The USA Machine Learning in Healthcare market is influenced by a mix of platform incumbents (EHR/cloud) and specialist ML vendors in imaging, clinical decision support, operational analytics, and life-sciences AI. This structure creates a “workflow plus data” competition: companies that can embed ML outputs directly into clinician/payer work queues—while meeting governance and evidence expectations—tend to scale faster than standalone analytics tools.
| Company | Est. year | HQ | Primary ML focus area | Integration surface | Evidence/validation posture | Deployment model | Enterprise scalability | Compliance readiness |
| Microsoft (Nuance) | 1975 (Microsoft) / Nuance 1992 | Redmond, WA / Burlington, MA | ~ | ~ | ~ | ~ | ~ | ~ |
| Epic Systems | 1979 | Verona, WI | ~ | ~ | ~ | ~ | ~ | ~ |
| Oracle Health (Cerner) | 1977 (Cerner) | Austin, TX / Kansas City area | ~ | ~ | ~ | ~ | ~ | ~ |
| NVIDIA | 1993 | Santa Clara, CA | ~ | ~ | ~ | ~ | ~ | ~ |
| Tempus | 2015 | Chicago, IL | ~ | ~ | ~ | ~ | ~ | ~ |
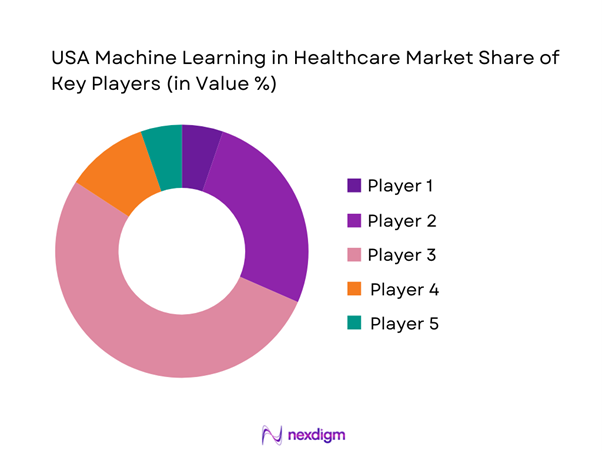
USA Machine Learning in Healthcare Market Analysis
Growth Drivers
EHR data liquidity and interoperability progress
U.S. healthcare ML adoption accelerates as clinical data becomes more portable across networks and care settings, expanding the “trainable” footprint of longitudinal records. At the national level, TEFCA exchange momentum is increasing: over ~ separate organizations have signed up to participate across ~ QHINs, representing more than ~ unique connections spanning clinicians, hospitals, clinics, post-acute providers, and public health authorities—materially expanding cross-network query/retrieve and event-based exchange opportunities for ML pipelines and real-world evidence loops. Parallel consolidation and scale effects are visible in the health information organization layer: a national survey identified ~ operational HIOs operating in ~ states and containing over ~ patient records (with duplication), underscoring both the scale and the normalization/identity-resolution challenges that ML vendors must handle to operationalize models across heterogeneous sources. These interoperability tailwinds matter because U.S. delivery is geographically fragmented yet economically massive: the U.S. economy is USD ~ trillion with a population of ~, enabling large multi-entity deployment footprints where ML value improves with broader data coverage (care transitions, medication reconciliation, longitudinal risk).
Imaging volume growth and radiologist productivity pressure
Clinical imaging remains the single largest ML commercialization corridor in U.S. healthcare because imaging workflows generate high-frequency digital artifacts and tightly defined endpoints (triage, detection, segmentation, quantification). The operational backdrop is scale: the American College of Radiology’s Dose Index Registry reported ~ imaging examinations in its dataset, reflecting the volume environment where radiology groups and hospitals prioritize automation, protocoling support, and prioritization tools to protect turnaround times and coverage models. On the regulatory supply side, U.S. commercialization is accelerating: the FDA authorized ~ AI/ML-enabled devices in a single year, with radiology continuing to dominate authorizations—evidence of both demand pull (workflow strain) and vendor push (productization of imaging ML). At the macro layer, the U.S. labor market and healthcare delivery system operate at a scale where even small productivity gains become financially meaningful: the U.S. unemployment rate is ~ and the economy is USD ~ trillion, creating persistent wage and staffing pressures for specialist-heavy services like radiology. Imaging ML adoption therefore tracks (i) high exam volumes, (ii) specialist productivity constraints, and (iii) a fast-moving FDA authorization pipeline that reduces procurement friction for hospital committees and enables enterprise rollouts.
Challenges
Clinical validation and evidence generation requirements
U.S. healthcare ML faces a higher “proof burden” than many digital markets because models influence clinical decisions, reimbursement, and regulated device pathways. The FDA’s device authorization tempo underscores both opportunity and compliance load: the FDA authorized ~ AI/ML-enabled devices in a single year, and researchers analyzing FDA authorizations documented an expanding pipeline that still requires disciplined clinical evaluation, predicate strategy, and post-market monitoring plans. Regulators are also formalizing lifecycle expectations: FDA materials on AI-enabled device software functions and Good Machine Learning Practice emphasize total product lifecycle controls and governance principles, expanding documentation, monitoring, and change-control expectations for deploy-and-learn systems in real clinical settings. For enterprises, this translates into higher costs of evidence generation (multi-site validation, subgroup analysis, drift monitoring), longer procurement cycles, and heavier clinician review. The macro operating environment—USD ~ trillion GDP and ~ population—drives scale, but also raises the stakes of adverse events and liability, which in turn increases the rigor demanded by health systems, payers, and regulators before enterprise-wide rollout.
Algorithm bias, fairness, and generalizability risks
Bias and generalizability are persistent U.S. ML constraints because deployment frequently spans heterogeneous patient populations, multi-payer benefit designs, and non-uniform data quality across institutions. One structural signal is that the FDA’s AI/ML device landscape is now measured in the hundreds to around a thousand depending on snapshot methodology—e.g., a JAMA Network Open analysis reported ~ authorized AI/ML devices and noted concentration in radiology devices (~)—meaning models are proliferating faster than health systems’ ability to standardize governance, subgroup evaluation, and monitoring across real-world cohorts. As model counts grow, so does the need for statistically adequate subgroup validation across a population of ~, where demographic, socioeconomic, and geographic variation is operationally meaningful and can drive distribution shift between training and deployment sites. The bias problem is also operational: cross-entity deployment expands faster when interoperability improves (e.g., TEFCA participation scaling), but that expansion increases heterogeneity and therefore the risk that site-specific coding practices, imaging protocols, or missingness patterns degrade model reliability without explicit recalibration and monitoring.
Opportunities
Federated learning and privacy-preserving analytics
U.S. healthcare is structurally positioned for privacy-preserving ML because the system is fragmented (many data owners) and privacy/cyber risk is high, which makes “move the model, not the data” architectures commercially attractive. Multi-site clinical research is already demonstrating the feasibility of federated approaches at scale: a Nature Communications study described federated learning using data from ~ sites across ~ continents for a cancer imaging use case, providing a proof pattern that can translate to U.S. provider consortia and multi-health-system deployments where data cannot easily be centralized. Public funding also supports data/AI infrastructure buildout: NIH announced it would invest USD ~ over ~ in the Bridge2AI program to accelerate AI use in biomedical and behavioral research—creating datasets, governance practices, and tooling that downstream vendors can leverage for more generalizable models and repeatable validation processes. With a USD ~ trillion economy and a population of ~, federated learning becomes a scalable pathway to expand training diversity while reducing privacy risk and minimizing cross-border or cross-state data transfer friction.
Multi-site enterprise AI deployments
The next growth wave in U.S. healthcare ML is enterprise, multi-site standardization—moving from point solutions to platform deployments across hospital networks, government systems, and payer-provider ecosystems. The addressable footprint is clear in federal health delivery: VHA operates ~ facilities including ~ medical centers and ~ outpatient sites, while the Military Health System supports ~ hospitals and clinics and serves ~ beneficiaries—enterprise-scale environments where model governance, monitoring, and integration frameworks can be replicated across sites once proven. Interoperability infrastructure growth (over ~ organizations participating in TEFCA across ~ QHINs, with ~ unique connections) increases the feasibility of cross-entity deployments that require external data signals (outside-lab results, outside imaging, transitions of care). In a macro environment of USD ~ trillion GDP, these multi-site deployments support “industrialization” of ML (shared MLOps, procurement leverage, common clinical governance), which can drive faster scaling than single-facility pilots even without relying on future-looking statistics.
Future Outlook
Over the next five to six years, the USA Machine Learning in Healthcare market is expected to expand rapidly as ML shifts from pilot projects to enterprise-scale standard operating capability. Growth will be driven by (i) scaling of workflow AI in documentation, coding, and care coordination, (ii) increasing use of ML in imaging and diagnostics triage, (iii) payer modernization for utilization management and fraud analytics, and (iv) accelerating life-sciences adoption for discovery and trials optimization. At the same time, procurement will harden around governance, monitoring, interoperability, and evidence requirements—rewarding vendors that can prove measurable operational and clinical impact.
Major Players
- Epic Systems
- Oracle Health
- Microsoft
- IBM
- Google Health / Google Cloud
- Amazon Web Services
- NVIDIA
- GE HealthCare
- Siemens Healthineers
- Philips
- Tempus AI
- Aidoc
- Viz.ai
- Abridge
Key Target Audience
- Hospital Systems & IDNs
- Healthcare Payers & Managed Care Organizations
- Pharmaceutical & Biotechnology Companies
- Medical Imaging Networks & Radiology Service Providers
- Healthcare IT & EHR/Workflow Platform Vendors
- Cloud/Hyperscaler Healthcare Business Units
- Investments and Venture Capitalist Firms
- Government and Regulatory Bodies
Research Methodology
Step 1: Identification of Key Variables
We build a U.S.-specific ecosystem map covering providers, payers, life sciences, EHR vendors, cloud platforms, and AI specialists. Secondary research consolidates regulatory, reimbursement adjacency, and procurement patterns to define variables such as deployment model, integration surface, evidence thresholds, and governance maturity.
Step 2: Market Analysis and Construction
We structure the market using a revenue pool approach across technology and end-user demand centers. Historical commercialization patterns are assessed through product launches, enterprise deployments, and category adoption signals, aligning them with workflow categories (clinical, operational, payer, life sciences).
Step 3: Hypothesis Validation and Expert Consultation
We validate hypotheses through structured interviews with provider informatics leaders, payer analytics heads, life-sciences data leaders, and vendor executives. CATI-style discussions test assumptions on ROI metrics, implementation timelines, integration barriers, and model monitoring requirements.
Step 4: Research Synthesis and Final Output
We synthesize findings into segment-level narratives and competitive benchmarks. The final output cross-verifies market direction with regulatory expectations, interoperability realities, and buyer decision pathways—ensuring the report reflects real procurement gates and scale-up constraints.
- Executive Summary
- Research Methodology (Market definitions and assumptions, abbreviations, inclusion and exclusion criteria, market engineering framework, primary interview mix by stakeholder, validation triangulation, pricing and monetization mapping, regulatory diligence checks, limitations)
- Definition and Scope
- Market Genesis and Inflection Points
- Business Cycle and Innovation Flywheel
- Healthcare AI Stack Overview
- Care Continuum Mapping
- USA-Specific Demand Context
- Growth Drivers
EHR data liquidity and interoperability progress
Imaging volume growth and radiologist productivity pressure
Clinical workforce shortages and automation demand
Payer focus on utilization management and claims integrity
Hospital throughput and length-of-stay optimization - Challenges
Clinical validation and evidence generation requirements
Algorithm bias, fairness, and generalizability risks
Interoperability and data standardization gaps
Clinician adoption and workflow disruption
Cybersecurity and patient data privacy risks - Opportunities
Federated learning and privacy-preserving analytics
Multi-site enterprise AI deployments
Specialty-specific AI solution bundles
Outcome-linked and shared-savings commercial models
Predictive and preventive care expansion - Trends
Foundation models in clinical text and imaging
Ambient clinical documentation and voice AI
Multimodal AI across EHR, imaging, and genomics
Agent-based workflow automation
Synthetic data generation for model training - Regulatory & Policy Landscape
- SWOT Analysis
- Stakeholder & Ecosystem Analysis
- Porter’s Five Forces Analysis
- Competitive Intensity & Ecosystem Mapping
- By Value, 2019–2024
- By Volume, 2019–2024
- By Average Contract Value and Average Revenue per Deployment, 2019–2024
- By Application (in Value %)
Clinical decision support
Medical imaging AI
Remote patient monitoring and predictive monitoring
Operational and workflow AI
Revenue cycle and coding automation
Clinical trials and real-world evidence analytics - By Technology Architecture (in Value %)
Classical machine learning models
Deep learning models
Natural language processing and large language models
Multimodal AI models
Time-series forecasting models
Graph-based machine learning models - By Connectivity Type (in Value %)
On-premise deployments
Private cloud deployments
Public cloud deployments
Hybrid deployments
Edge or on-device inference - By End-Use Industry (in Value %)
Integrated delivery networks and health systems
Community and regional hospitals
Specialty clinics
Health insurance payers and managed care organizations
Pharmaceutical and biotechnology companies - By Region (in Value %)
Northeast
Midwest
South
West
- Competitive universe and positioning map
- Market share estimation framework
- Cross Comparison Parameters (FDA clearance footprint depth, EHR and PACS integration breadth, clinical evidence strength, enterprise deployment scalability, MLOps maturity and drift controls, data modality coverage, security and compliance readiness, commercial model flexibility)
- Competitive moats and vulnerabilities
- SWOT Analysis of Key Players
- Partnerships and alliance landscape
- Detailed Profiles of Major Companies
Epic Systems
Oracle Health (Cerner)
Microsoft (Nuance)
Google Cloud and DeepMind Health collaborations
GE HealthCare
Siemens Healthineers
Philips
NVIDIA
Tempus
Flatiron Health
Viz.ai
Aidoc
PathAI
IBM
SAS
- Provider Buying Behavior
- Payer Buying Behavior
- Pharma/CRO Buying Behavior
- Points & Unmet Needs
- Implementation & Change Management
- By Value, 2025–2030
- By Volume, 2025–2030
- By Average Contract Value and Average Revenue per Deployment, 2025–2030